Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir
- PMID: 15328119
- PMCID: PMC514741
- DOI: 10.1128/AAC.48.9.3516-3522.2004
Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir
Abstract
To improve the oral bioavailability of cidofovir (CDV), a series of ether lipid ester prodrugs were synthesized and evaluated for activity against murine cytomegalovirus (MCMV) infection. Four of these analogs, hexadecyloxypropyl (HDP)-CDV, octadecyloxyethyl (ODE)-CDV, oleyloxyethyl (OLE)-CDV, and oleyloxypropyl (OLP)-CDV, were found to have greater activity than CDV against human CMV and MCMV in vitro. The efficacy of oral treatment with these compounds against MCMV infections in BALB/c mice was then determined. Treatment with HDP-CDV, ODE-CDV, OLE-CDV, or OLP-CDV at 2.0 to 6.7 mg/kg of body weight provided significant protection when daily treatments were initiated 24 to 48 h after viral inoculation. Additionally, HDP-CDV or ODE-CDV administered twice weekly or as a single dose of 1.25 to 10 mg/kg was effective in reducing mortality when treatment was initiated at 24 h, 48 h, or, in some cases, 72 h after viral inoculation. In animals treated daily with HDP-CDV or ODE-CDV, virus titers in lung, liver, spleen, kidney, pancreas, salivary gland, and blood were reduced 3 to 5 log(10)-fold, which was comparable to CDV given intraperitoneally. These results indicated that HDP-CDV or ODE-CDV given orally was as effective as parenteral CDV for the treatment of experimental MCMV infection and suggest that further evaluation for use in CMV infections in humans is warranted.
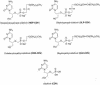
Figures

Similar articles
-
Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus (MCMV) or human cytomegalovirus (HCMV) in animal models.Antiviral Res. 2008 Aug;79(2):133-5. doi: 10.1016/j.antiviral.2008.01.155. Epub 2008 Feb 22. Antiviral Res. 2008. PMID: 18336926 Free PMC article.
-
Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir.Antimicrob Agents Chemother. 2004 Feb;48(2):404-12. doi: 10.1128/AAC.48.2.404-412.2004. Antimicrob Agents Chemother. 2004. PMID: 14742188 Free PMC article.
-
Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection.J Infect Dis. 2004 Aug 1;190(3):499-503. doi: 10.1086/421912. Epub 2004 Jul 1. J Infect Dis. 2004. PMID: 15243923
-
Cidofovir.Drugs. 1996 Aug;52(2):225-230; discussion 231. doi: 10.2165/00003495-199652020-00006. Drugs. 1996. PMID: 8841740 Review.
-
A preclinical and clinical overview of the nucleotide-based antiviral agent cidofovir (HPMPC).Adv Exp Med Biol. 1996;394:105-15. doi: 10.1007/978-1-4757-9209-6_12. Adv Exp Med Biol. 1996. PMID: 8815677 Review. No abstract available.
Cited by
-
Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus (MCMV) or human cytomegalovirus (HCMV) in animal models.Antiviral Res. 2008 Aug;79(2):133-5. doi: 10.1016/j.antiviral.2008.01.155. Epub 2008 Feb 22. Antiviral Res. 2008. PMID: 18336926 Free PMC article.
-
Antiviral Therapies for Herpesviruses: Current Agents and New Directions.Clin Ther. 2018 Aug;40(8):1282-1298. doi: 10.1016/j.clinthera.2018.07.006. Epub 2018 Aug 10. Clin Ther. 2018. PMID: 30104016 Free PMC article. Review.
-
Cytomegalovirus antivirals and development of improved animal models.Expert Opin Drug Metab Toxicol. 2011 Oct;7(10):1245-65. doi: 10.1517/17425255.2011.613824. Epub 2011 Sep 1. Expert Opin Drug Metab Toxicol. 2011. PMID: 21883024 Free PMC article. Review.
-
Phosphonate prodrugs: an overview and recent advances.Future Med Chem. 2019 Jul;11(13):1625-1643. doi: 10.4155/fmc-2018-0591. Future Med Chem. 2019. PMID: 31469328 Free PMC article. Review.
-
Rethinking Remdesivir: Synthesis, Antiviral Activity, and Pharmacokinetics of Oral Lipid Prodrugs.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0115521. doi: 10.1128/AAC.01155-21. Epub 2021 Jul 26. Antimicrob Agents Chemother. 2021. PMID: 34310217 Free PMC article.
References
-
- Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. - PubMed
-
- Balfour, H. H., Jr., B. A. Chace, J. T. Stapleton, R. L. Simmons, and D. S. Fryd. 1989. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. N. Engl. J. Med. 320:1381-1387. - PubMed
-
- Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. - PMC - PubMed
-
- Bidanset, D. J., J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2004. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infections. J. Infect. Dis. 190:499-503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

