Disposition of posaconazole following single-dose oral administration in healthy subjects
- PMID: 15328123
- PMCID: PMC514780
- DOI: 10.1128/AAC.48.9.3543-3551.2004
Disposition of posaconazole following single-dose oral administration in healthy subjects
Abstract
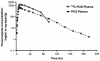
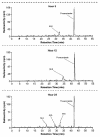
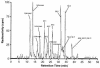
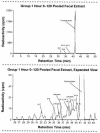
Posaconazole is a potent, broad-spectrum triazole antifungal agent currently in clinical development for the treatment of refractory invasive fungal infections. Eight healthy male subjects received a single 399-mg (81.7 microCi) oral dose of [(14)C]posaconazole after consuming a high-fat breakfast. Urine, feces, and blood samples were collected for up to 336 h postdose and assayed for total radioactivity; plasma and urine samples were also assayed for parent drug. Posaconazole was orally bioavailable, with a median maximum posaconazole concentration in plasma achieved by 10 h postdose. Thereafter, posaconazole was slowly eliminated, with a mean half-life of 20 h. The greatest peak in the radioactivity profile of pooled plasma extracts was due to posaconazole, with smaller peaks due to a monoglucuronide, a diglucuronide, and a smaller fragment of the molecule. The mean total amount of radioactivity recovered was 91.1%; the cumulative excretion of radioactivity in feces and in urine was 76.9 and 14.0% of the dose, respectively. Most of the fecal radioactivity was associated with posaconazole, which accounted for 66.3% of the administered dose; however, urine contained only trace amounts of unchanged posaconazole. The radioactivity profile of pooled urine extracts included two monoglucuronide conjugates and a diglucuronide conjugate of posaconazole. These observations suggest that oxidative (phase 1) metabolism by cytochrome P450 isoforms represents only a minor route of elimination for posaconazole, and therefore cytochrome P450-mediated drug interactions should have a limited potential to impact posaconazole pharmacokinetics.
Figures



Similar articles
-
Disposition and biotransformation of the antiretroviral drug nevirapine in humans.Drug Metab Dispos. 1999 Aug;27(8):895-901. Drug Metab Dispos. 1999. PMID: 10421616 Clinical Trial.
-
Characterization of the Pharmacokinetics and Mass Balance of a Single Oral Dose of Trofinetide in Healthy Male Subjects.Clin Drug Investig. 2024 Jan;44(1):21-33. doi: 10.1007/s40261-023-01322-2. Epub 2023 Nov 28. Clin Drug Investig. 2024. PMID: 38017349 Free PMC article. Clinical Trial.
-
Pharmacokinetics of a single dose of the antifungal posaconazole as oral suspension in subjects with hepatic impairment.Curr Med Res Opin. 2010 Jan;26(1):1-7. doi: 10.1185/03007990903364657. Curr Med Res Opin. 2010. PMID: 19886860
-
Posaconazole: clinical pharmacology and potential for management of fungal infections.Expert Rev Anti Infect Ther. 2005 Aug;3(4):467-87. doi: 10.1586/14787210.3.4.467. Expert Rev Anti Infect Ther. 2005. PMID: 16107193 Review.
-
Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent.Clin Infect Dis. 2007 Dec 15;45(12):1610-7. doi: 10.1086/523576. Clin Infect Dis. 2007. PMID: 18190324 Review.
Cited by
-
Successful use of posaconazole to treat invasive cutaneous fungal infection in a liver transplant patient on sirolimus.Can J Infect Dis Med Microbiol. 2012 Summer;23(2):e44-7. doi: 10.1155/2012/272197. Can J Infect Dis Med Microbiol. 2012. PMID: 23730320 Free PMC article.
-
Clinical Pharmacokinetics of Second-Generation Triazoles for the Treatment of Invasive Aspergillosis and Candidiasis.Eur J Drug Metab Pharmacokinet. 2019 Apr;44(2):139-157. doi: 10.1007/s13318-018-0513-7. Eur J Drug Metab Pharmacokinet. 2019. PMID: 30284178 Review.
-
Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene.Antimicrob Agents Chemother. 2010 Feb;54(2):860-5. doi: 10.1128/AAC.00931-09. Epub 2009 Nov 16. Antimicrob Agents Chemother. 2010. PMID: 19917751 Free PMC article.
-
Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo.Clin Pharmacol Ther. 2008 Jan;83(1):77-85. doi: 10.1038/sj.clpt.6100230. Epub 2007 May 9. Clin Pharmacol Ther. 2008. PMID: 17495874 Free PMC article. Clinical Trial.
-
Simultaneous quantitation of five triazole anti-fungal agents by paper spray-mass spectrometry.Clin Chem Lab Med. 2020 Apr 28;58(5):836-846. doi: 10.1515/cclm-2019-0895. Clin Chem Lab Med. 2020. PMID: 31926066 Free PMC article.
References
-
- Barchiesi, F., A. M. Schimizzi, F. Caselli, D. Giannini, V. Camiletti, B. Fileni, A. Giacometti, L. F. Di Francesco, and G. Scalise. 2001. Activity of the new antifungal triazole, posaconazole, against Cryptococcus neoformans. J. Antimicrob. Chemother. 48:769-773. - PubMed
-
- Ghosal, A., N. Hapangama, Y. Yuan, J. Achanfuo-Yeboah, R. M. Iannucci, S. Chowdhury, K. B. Alton, J. E. Patrick, and S. Zbaida. 2004. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab. Dispos. 32:267-271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

