Thioredoxin can influence gene expression by affecting gyrase activity
- PMID: 15328368
- PMCID: PMC516065
- DOI: 10.1093/nar/gkh794
Thioredoxin can influence gene expression by affecting gyrase activity
Abstract
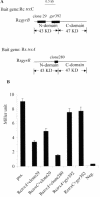
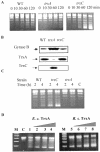
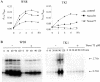
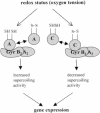
The expression of many genes of facultatively photosynthetic bacteria of the genus Rhodobacter is controlled by the oxygen tension. Among these are the genes of the puf and puc operons, which encode proteins of the photosynthetic apparatus. Previous results revealed that thioredoxins are involved in the regulated expression of these operons, but it remained unsolved as to the mechanisms by which thioredoxins affect puf and puc expression. Here we show that reduced TrxA of Rhodobacter capsulatus and Rhodobacter sphaeroides and oxidized TrxC of R.capsulatus interact with DNA gyrase and alter its DNA supercoiling activity. While TrxA enhances supercoiling, TrxC exerts a negative effect on this activity. Furthermore, inhibition of gyrase activity strongly reduces puf and puc expression. Our results reveal a new signaling pathway by which oxygen can affect the expression of bacterial genes.
Figures




References
-
- Laurent T.C., Moore,E.C. and Reichard,P. (1964) Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli. J. Biol. Chem., 239, 3436–3444. - PubMed
-
- Holmgren A. (1984) Enzymatic reduction–oxidation of protein disulfides by thioredoxin. Methods Enzymol., 107, 295–300. - PubMed
-
- Carmel-Harel O. and Storz,G. (2000) Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol., 54, 439–461. - PubMed
-
- Ritz D. and Beckwith,J. (2001) Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol., 55, 21–48. - PubMed
-
- Schulze-Osthoff K., Schenk,H. and Droge,W. (1995) Effects of thioredoxin on activation of transcription factor NF-kappa B. Methods Enzymol., 252, 253–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

