Multiple pathways process stalled replication forks
- PMID: 15328417
- PMCID: PMC516472
- DOI: 10.1073/pnas.0401586101
Multiple pathways process stalled replication forks
Abstract
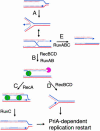
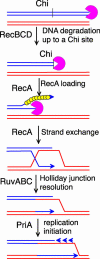
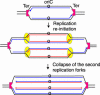
Impairment of replication fork progression is a serious threat to living organisms and a potential source of genome instability. Studies in prokaryotes have provided evidence that inactivated replication forks can restart by the reassembly of the replication machinery. Several strategies for the processing of inactivated replication forks before replisome reassembly have been described. Most of these require the action of recombination proteins, with different proteins being implicated, depending on the cause of fork arrest. The action of recombination proteins at blocked forks is not necessarily accompanied by a strand-exchange reaction and may prevent rather than repair fork breakage. These various restart pathways may reflect different structures at stalled forks. We review here the different strategies of fork processing elicited by different kinds of replication impairments in prokaryotes and the variety of roles played by recombination proteins in these processes.
Copyright 2004 The National Academy of Sciencs of the USA
Figures


References
-
- Xu, L. & Marians, K. J. (2003) Mol. Cell 11, 817–826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

