Antitumour efficacy of VEGFR2 tyrosine kinase inhibitor correlates with expression of VEGF and its receptor VEGFR2 in tumour models
- PMID: 15328520
- PMCID: PMC2409895
- DOI: 10.1038/sj.bjc.6602109
Antitumour efficacy of VEGFR2 tyrosine kinase inhibitor correlates with expression of VEGF and its receptor VEGFR2 in tumour models
Abstract
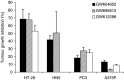
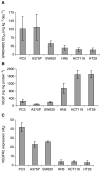
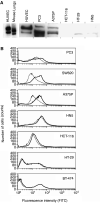
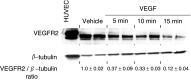
During the development of indazolylpyrimidines as novel and potent inhibitors of vascular endothelial growth factor (VEGF) receptor-2 (VEGFR2) tyrosine kinase, we observed that some human tumour xenografts are more sensitive to VEGFR2 kinase inhibitors than others. A better understanding of the basis for this differential response may help to identify a predictive marker that would greatly aid in the identification of a suitable patient population for treatment. One representative compound from the indazolylpyrimidine series is GW654652 that inhibited all three VEGFRs with similar potency. The inhibition of VEGFR2 kinase by GW654652 was about 150 to >8800 more potent than the inhibition of eight other kinases tested. GW654652 inhibited VEGF- and bFGF-induced proliferation in endothelial cells with an IC(50) of 110 and 1980 nM, respectively, and has good pharmacokinetic profile in mouse and dog. We investigated the association between VEGF and VEGFR2 expression and the antitumour efficacy of GW654652, in various xenograft models. Statistically significant associations were observed between the antitumour efficacy of GW654652 in xenografts and VEGF protein (P=0.005) and VEGFR2 expression (P=0.041). The oral dose of GW654652 producing 50% inhibition of tumour growth (ED(50)) decreased with increasing levels of VEGF (r=-0.94); and, in contrast, the ED(50) increased with the increased expression of VEGFR2 (r=0.82). These results are consistent with the observed inverse correlation between VEGF and VEGFR2 expression in tumours. These findings support the hypothesis that VEGF and VEGFR2 expression by tumours may predict the therapeutic outcome of VEGFR kinase inhibitors.
Figures
Similar articles
-
Inhibition of VEGF receptors significantly impairs mammary cancer growth in C3(1)/Tag transgenic mice through antiangiogenic and non-antiangiogenic mechanisms.Oncogene. 2005 Jan 27;24(5):790-800. doi: 10.1038/sj.onc.1208221. Oncogene. 2005. PMID: 15592523
-
GW654652, the pan-inhibitor of VEGF receptors, blocks the growth and migration of multiple myeloma cells in the bone marrow microenvironment.Blood. 2004 May 1;103(9):3474-9. doi: 10.1182/blood-2003-10-3527. Epub 2003 Nov 26. Blood. 2004. PMID: 14644994
-
CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models.Cancer Res. 2003 Sep 15;63(18):5978-91. Cancer Res. 2003. PMID: 14522925
-
Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis.J Biochem Mol Biol. 2006 Sep 30;39(5):469-78. doi: 10.5483/bmbrep.2006.39.5.469. J Biochem Mol Biol. 2006. PMID: 17002866 Review.
-
Vascular endothelial growth factor (VEGF)-Receptor2: its biological functions, major signaling pathway, and specific ligand VEGF-E.Endothelium. 2006 Mar-Apr;13(2):63-9. doi: 10.1080/10623320600697955. Endothelium. 2006. PMID: 16728325 Review.
Cited by
-
Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: an immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors.Am J Surg Pathol. 2012 Apr;36(4):629-39. doi: 10.1097/PAS.0b013e318243555b. Am J Surg Pathol. 2012. PMID: 22314185 Free PMC article.
-
Anti-angiogenic and anti-tumor effects of TAK-593, a potent and selective inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptor tyrosine kinase.Cancer Sci. 2013 Apr;104(4):486-94. doi: 10.1111/cas.12101. Epub 2013 Feb 18. Cancer Sci. 2013. PMID: 23305239 Free PMC article.
-
YL529, a novel, orally available multikinase inhibitor, potently inhibits angiogenesis and tumour growth in preclinical models.Br J Pharmacol. 2013 Aug;169(8):1766-80. doi: 10.1111/bph.12216. Br J Pharmacol. 2013. PMID: 23594209 Free PMC article.
-
Design, Synthesis and Biological Activity Evaluation of S-Substituted 1H-5-Mercapto-1,2,4-Triazole Derivatives as Antiproliferative Agents in Colorectal Cancer.Front Chem. 2018 Aug 23;6:373. doi: 10.3389/fchem.2018.00373. eCollection 2018. Front Chem. 2018. PMID: 30234098 Free PMC article.
-
High Density Lipoprotein Nanoparticles Deliver RNAi to Endothelial Cells to Inhibit Angiogenesis.Part Part Syst Charact. 2014 Nov 1;31(11):1141-1150. doi: 10.1002/ppsc.201400036. Part Part Syst Charact. 2014. PMID: 25400330 Free PMC article.
References
-
- Aguayo A, O'Brien S, Keating M, Manshouri T, Gidel C, Barlogie B, Beran M, Koller C, Kantarjian H, Albitar M (2000) Clinical relevance of intracellular vascular endothelial growth factor levels in B-cell chronic lymphocytic leukemia. Blood 96: 768–770 - PubMed
-
- Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N, Wieand HS (2003) Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes' B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol 21: 241–250 - PubMed
-
- Alt FW, Kellems RE, Bertino JR, Schimke RT (1978) Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem 253: 1357–1370 - PubMed
-
- Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3: 401–410 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

