Pharmacokinetics of the nonsteroidal steroid sulphatase inhibitor 667 COUMATE and its sequestration into red blood cells in rats
- PMID: 15328524
- PMCID: PMC2409900
- DOI: 10.1038/sj.bjc.6602130
Pharmacokinetics of the nonsteroidal steroid sulphatase inhibitor 667 COUMATE and its sequestration into red blood cells in rats
Abstract
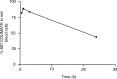
Breast cancer is a major cause of mortality in Western countries and there is an urgent requirement for novel treatment strategies. The nonsteroidal sulphatase inhibitor 667 COUMATE inhibits hepatic steroid sulphatase and growth of oestrone sulphate stimulated tumours in the nitrosomethylurea-induced rat mammary model. Other compounds that contain an aryl sulphamate moiety, for example, oestrone-3-O-sulphamate, are sequestered into red blood cells (RBCs). The aims of this study were to determine the pharmacokinetics of 667 COUMATE and to investigate its sequestration into RBCs. We administered a single p.o. or i.v. dose (10 mg kg(-1)) of 667 COUMATE to rats and used a high-performance liquid chromatography method to measure the levels of the agent and its putative metabolites in plasma. 667 COUMATE had a bioavailability of 95% and could be detected in plasma for up to 8 h. Using two independent analytical methods, we demonstrated that 667 COUMATE is sequestered by RBCs both ex vivo and in vivo. Previous investigations have revealed that 667 COUMATE is rapidly degraded in plasma ex vivo. In this study, we demonstrate that 667 COUMATE is stabilised due to its sequestration into RBCs. In conclusion, the pharmacological efficacy and high oral bioavailability of 667 COUMATE may be partly a consequence of the ability of RBCs to both protect the agent from metabolic degradation and facilitate its transport to tissues. These data support the further clinical evaluation of this novel endocrine therapeutic agent.
Figures



References
-
- Abbate F, Winum J-Y, Potter BVL, Casini A, Montero J-L, Scozzafava A, Supuran CT (2004) Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isoenzyme II with EMATE, a dual inhibitor of carbonic anhydrases and steroid sulfatases. Bioorg Med Chem Lett 14: 337–341 - PubMed
-
- Baerlocher GM, Beer JM, Owen GR, Meiselman HJ, Reinhart WH (1997) The antineoplastic drug 5-fluororacil produces echinocytosis and affects blood rheology. Br J Haemotol 99: 426–433 - PubMed
-
- Bernstein L, Ross RK (1993) Endogenous hormones and breast cancer. Epidemiol Rev 15: 48–65 - PubMed
-
- Casini A, Antel J, Abbate F, Scozzafava A, David S, Waldeck H, Schafer S, Supuran CT (2003) Carbonic anhydrase inhibitors: SAR and X-ray crystallographic study of the interaction of sugar sulphamates/sulfonamides with isozymes I, II and IV. Bioorg Med Chem Lett 13: 841–845 - PubMed
-
- Elger W, Palme H-J, Schwarz S (1998) Novel estrogen sulphamates: a new approach to oral hormone therapy. Exp Opin Invest Drugs 7: 575–589 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

