Loss of skeletal muscle HIF-1alpha results in altered exercise endurance
- PMID: 15328538
- PMCID: PMC514537
- DOI: 10.1371/journal.pbio.0020288
Loss of skeletal muscle HIF-1alpha results in altered exercise endurance
Abstract
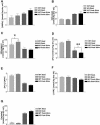
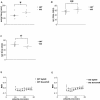
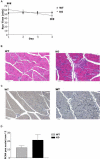
The physiological flux of oxygen is extreme in exercising skeletal muscle. Hypoxia is thus a critical parameter in muscle function, influencing production of ATP, utilization of energy-producing substrates, and manufacture of exhaustion-inducing metabolites. Glycolysis is the central source of anaerobic energy in animals, and this metabolic pathway is regulated under low-oxygen conditions by the transcription factor hypoxia-inducible factor 1alpha (HIF-1alpha). To determine the role of HIF-1alpha in regulating skeletal muscle function, we tissue-specifically deleted the gene encoding the factor in skeletal muscle. Significant exercise-induced changes in expression of genes are decreased or absent in the skeletal-muscle HIF-1alpha knockout mice (HIF-1alpha KOs); changes in activities of glycolytic enzymes are seen as well. There is an increase in activity of rate-limiting enzymes of the mitochondria in the muscles of HIF-1alpha KOs, indicating that the citric acid cycle and increased fatty acid oxidation may be compensating for decreased flow through the glycolytic pathway. This is corroborated by a finding of no significant decreases in muscle ATP, but significantly decreased amounts of lactate in the serum of exercising HIF-1alpha KOs. This metabolic shift away from glycolysis and toward oxidation has the consequence of increasing exercise times in the HIF-1alpha KOs. However, repeated exercise trials give rise to extensive muscle damage in HIF-1alpha KOs, ultimately resulting in greatly reduced exercise times relative to wild-type animals. The muscle damage seen is similar to that detected in humans in diseases caused by deficiencies in skeletal muscle glycogenolysis and glycolysis. Thus, these results demonstrate an important role for the HIF-1 pathway in the metabolic control of muscle function.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
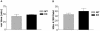
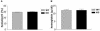
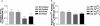
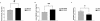

Similar articles
-
High-intensity interval training-induced metabolic adaptation coupled with an increase in Hif-1α and glycolytic protein expression.J Appl Physiol (1985). 2015 Dec 1;119(11):1297-302. doi: 10.1152/japplphysiol.00499.2015. Epub 2015 Oct 1. J Appl Physiol (1985). 2015. PMID: 26429867
-
HIF-1alpha in endurance training: suppression of oxidative metabolism.Am J Physiol Regul Integr Comp Physiol. 2007 Nov;293(5):R2059-69. doi: 10.1152/ajpregu.00335.2007. Epub 2007 Sep 12. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17855495
-
Skeletal muscle hypoxia-inducible factor-1 and exercise.Exp Physiol. 2016 Jan;101(1):28-32. doi: 10.1113/EP085318. Epub 2015 Oct 15. Exp Physiol. 2016. PMID: 26391197 Review.
-
The role of HIF-1 in hypoxic response in the skeletal muscle.Adv Exp Med Biol. 2007;618:229-44. doi: 10.1007/978-0-387-75434-5_18. Adv Exp Med Biol. 2007. PMID: 18269201 Review.
-
TNF-α-induced NF-κB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1α.Endocrinology. 2015 May;156(5):1770-81. doi: 10.1210/en.2014-1591. Epub 2015 Feb 24. Endocrinology. 2015. PMID: 25710281
Cited by
-
Carotenoid transporter CD36 expression depends on hypoxia-inducible factor-1α in mouse soleus muscles.J Clin Biochem Nutr. 2022 Sep;71(2):112-121. doi: 10.3164/jcbn.21-163. Epub 2022 May 26. J Clin Biochem Nutr. 2022. PMID: 36213788 Free PMC article.
-
Genes Whose Gain or Loss-of-Function Increases Endurance Performance in Mice: A Systematic Literature Review.Front Physiol. 2019 Mar 22;10:262. doi: 10.3389/fphys.2019.00262. eCollection 2019. Front Physiol. 2019. PMID: 30967789 Free PMC article. Review.
-
Human high-altitude adaptation: forward genetics meets the HIF pathway.Genes Dev. 2014 Oct 15;28(20):2189-204. doi: 10.1101/gad.250167.114. Genes Dev. 2014. PMID: 25319824 Free PMC article. Review.
-
Integrative Analysis of ATAC-Seq and RNA-Seq Identifies Key Genes Affecting Muscle Development in Ningxiang Pigs.Int J Mol Sci. 2025 Mar 14;26(6):2634. doi: 10.3390/ijms26062634. Int J Mol Sci. 2025. PMID: 40141276 Free PMC article.
-
The Impact of Exercise on Immunity, Metabolism, and Atherosclerosis.Int J Mol Sci. 2023 Feb 8;24(4):3394. doi: 10.3390/ijms24043394. Int J Mol Sci. 2023. PMID: 36834808 Free PMC article. Review.
References
-
- Argov Z, Bank WJ, Maris J, Leigh JS, Chance B. Muscle energy metabolism in human phosphofructokinase deficiency as recorded by 31P nuclear magnetic resonance spectroscopy. Ann Neurol. 1987;22:46–51. - PubMed
-
- Bancroft JD, Stevens A. New York: Churchill Livingstone; 1996. Theory and practice of histological techniques, 4th ed; 766 pp.
-
- Bergmeyer HU. New York: Academic Press; 1974. Methods of enzymatic analysis; 2302 pp.
-
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

