Epidermal growth factor and interleukin-1beta utilize divergent signaling pathways to synergistically upregulate cyclooxygenase-2 gene expression in human amnion-derived WISH cells
- PMID: 15329330
- PMCID: PMC1389598
- DOI: 10.1095/biolreprod.104.030841
Epidermal growth factor and interleukin-1beta utilize divergent signaling pathways to synergistically upregulate cyclooxygenase-2 gene expression in human amnion-derived WISH cells
Abstract
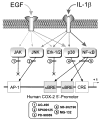
In human parturition, uterotonic prostaglandins (PGs) arise predominantly via increased expression of cyclooxygenase-2 (COX-2 [also known as prostaglandin synthase 2]) within intrauterine tissues. Interleukin-1 (IL-1) and epidermal growth factor (EGF), both inducers of COX-2 transcription, are among numerous factors that accumulate within amniotic fluid with advancing gestation. It was previously demonstrated that EGF could potentiate IL-1beta-driven PGE(2) production in amnion and amnion-derived (WISH) cells. To define the mechanism for this observation, we hypothesized that EGF and IL-1beta might exhibit synergism in regulating COX-2 gene expression. In WISH cells, combined treatment with EGF and IL-1beta resulted in a greater-than-additive increase in COX-2 mRNA relative to challenge with either agent independently. Augmentation of IL-1beta-induced transactivation by EGF was not observed in cells harboring reporter plasmids bearing nuclear factor-kappa B (NFkappaB) regulatory elements alone, but was evident when a fragment (-891/ +9) of the COX-2 gene 5'-promoter was present. Both agents transiently activated intermediates of multiple signaling pathways potentially involved in the regulation of COX-2 gene expression. The 26 S proteasome inhibitor, MG-132, selectively abrogated IL-1beta-driven NFkappaB activation and COX-2 mRNA expression. Only pharmacologic blockade of the p38 mitogen-activated protein kinase eliminated COX-2 expression following EGF stimulation. We conclude that EGF and IL-1beta appear to signal through different signaling cascades leading to COX-2 gene expression. IL-1beta employs the NFkappaB pathway predominantly, while the spectrum of EGF signaling is broader and includes p38 kinase. The synergism observed between IL-1beta and EGF does not rely on augmented NFkappaB function, but rather, occurs through differential use of independent response elements within the COX-2 promoter.
Figures

Similar articles
-
Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2.J Clin Endocrinol Metab. 2002 Jul;87(7):3263-73. doi: 10.1210/jcem.87.7.8594. J Clin Endocrinol Metab. 2002. PMID: 12107235
-
Nuclear factor kappa B activation and regulation of cyclooxygenase type-2 expression in human amnion mesenchymal cells by interleukin-1beta.Biol Reprod. 2002 Jun;66(6):1667-71. doi: 10.1095/biolreprod66.6.1667. Biol Reprod. 2002. PMID: 12021045
-
Involvement of tyrosine kinases on cyclooxygenase expression and prostaglandin E2 production in human gingival fibroblasts stimulated with interleukin-1beta and epidermal growth factor.Biochem Biophys Res Commun. 1999 Apr 13;257(2):528-32. doi: 10.1006/bbrc.1999.0523. Biochem Biophys Res Commun. 1999. PMID: 10198245
-
Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells.J Steroid Biochem Mol Biol. 2002 Feb;80(2):203-12. doi: 10.1016/s0960-0760(01)00187-x. J Steroid Biochem Mol Biol. 2002. PMID: 11897504 Review.
-
IL-1beta during in vitro decidualization in primate.J Reprod Immunol. 2002 May-Jun;55(1-2):35-47. doi: 10.1016/s0165-0378(01)00141-3. J Reprod Immunol. 2002. PMID: 12062820 Review.
Cited by
-
Inflammatory gene regulatory networks in amnion cells following cytokine stimulation: translational systems approach to modeling human parturition.PLoS One. 2011;6(6):e20560. doi: 10.1371/journal.pone.0020560. Epub 2011 Jun 2. PLoS One. 2011. PMID: 21655103 Free PMC article.
-
Cooperative effects of sequential PGF2α and IL-1β on IL-6 and COX-2 expression in human myometrial cells†.Biol Reprod. 2019 May 1;100(5):1370-1385. doi: 10.1093/biolre/ioz029. Biol Reprod. 2019. PMID: 30794283 Free PMC article.
-
The anthraquinone derivative Emodin ameliorates neurobehavioral deficits of a rodent model for schizophrenia.J Neural Transm (Vienna). 2008;115(3):521-30. doi: 10.1007/s00702-007-0867-5. Epub 2008 Feb 26. J Neural Transm (Vienna). 2008. PMID: 18301953
-
Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells.PLoS One. 2011 Apr 6;6(4):e18562. doi: 10.1371/journal.pone.0018562. PLoS One. 2011. PMID: 21494638 Free PMC article.
-
Modulation of cytokine-induced cyclooxygenase 2 expression by PPARG ligands through NFkappaB signal disruption in human WISH and amnion cells.Biol Reprod. 2005 Sep;73(3):527-35. doi: 10.1095/biolreprod.104.039032. Epub 2005 Apr 20. Biol Reprod. 2005. PMID: 15843495 Free PMC article.
References
-
- Kniss DA, Iams JD. Regulation of parturition update. Endocrine and paracrine effectors of term and preterm labor. Clin Perinatol. 1998;25:819–836. - PubMed
-
- Kniss DA. Cyclooxygenases in reproductive medicine and biology. J Soc Gynecol Invest. 1999;6:285–292. - PubMed
-
- Johnson RF, Mitchell CM, Giles WB, Walters WA, Zakar T. The in vivo control of prostaglandin H synthase-2 messenger ribonucleic acid expression in the human amnion at parturition. J Clin Endocrinol Metab. 2002;87:2816–2823. - PubMed
-
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition—a review. Placenta. 2003;24(suppl A):S33–S46. - PubMed
-
- Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

