Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats
- PMID: 15329395
- PMCID: PMC6729639
- DOI: 10.1523/JNEUROSCI.1312-04.2004
Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats
Abstract
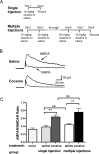
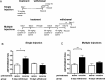
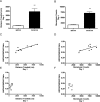
The initiation of the psychostimulant sensitization process depends on the mesolimbic system, which projects from the ventral tegmental area (VTA) to the nucleus accumbens. Although such initiation is primarily dependent on glutamatergic activity in VTA neurons, the exact role VTA excitatory synapses play in this process is poorly understood. Here, we examine the effects of repeated in vivo injections of cocaine on the magnitude and duration of the increase in strength at VTA excitatory synapses reported previously to be elicited by a single in vivo exposure to cocaine (Ungless et al., 2001; Saal et al., 2003). We also compare the synaptic modifications induced by cocaine with its effects on locomotor activity. Surprisingly, repeated cocaine exposure potentiated the ratio of AMPA receptor-mediated to NMDA receptor-mediated EPSCs to a similar extent and duration as a single in vivo cocaine exposure. In naive animals, the magnitude of the cocaine-induced locomotor activity after a single injection of cocaine correlated with the magnitude of the accompanying synaptic enhancement. This correlation was lost on the seventh day of repeated cocaine administration, as well as when a challenge injection was given 10 d after the cessation of repeated cocaine administration. These results suggest that the cocaine-induced synaptic plasticity at VTA excitatory synapses is transient, and its duration depends on the last exposure to cocaine. Furthermore, chronic cocaine exposure disrupts the normal, presumably adaptive relationship between synaptic enhancement in the VTA and behavior.
Figures




References
-
- Ackerman JM, White FJ (1990) A10 somatodendritic dopamine autoreceptor sensitivity following withdrawal from repeated cocaine treatment. Neurosci Lett 117: 181-187. - PubMed
-
- Bjijou Y, Stinus L, Moal ML, Cador M (1996) Evidence for selective involvement of dopamine D1 receptors of the ventral tegmental area in the behavioral sensitization induced by intra-ventral tegmental area injections of d-amphetamine. J Pharmacol Exp Ther 277: 1177-1187. - PubMed
-
- Cornish JL, Kalivas PW (2001) Repeated cocaine administration into the rat ventral tegmental area produces behavioral sensitization to a systemic cocaine challenge. Behav Brain Res 126: 205-209. - PubMed
-
- Cull-Candy S, Brickley S, Farrant M (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11: 327-335. - PubMed
-
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84: 745-755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
