Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles
- PMID: 15329415
- PMCID: PMC516571
- DOI: 10.1073/pnas.0405398101
Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles
Abstract
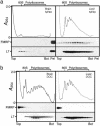
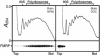
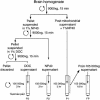
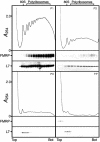
Fragile X syndrome is caused by the absence of the fragile X mental retardation protein (FMRP). This RNA-binding protein is widely expressed in human and mouse tissues, and it is particularly abundant in the brain because of its high expression in neurons, where it localizes in the cell body and in granules throughout dendrites. Although FMRP is thought to regulate trafficking of repressed mRNA complexes and to influence local protein synthesis in synapses, it is not known whether it has additional functions in the control of translation in the cell body. Here, we have used recently developed approaches to investigate whether FMRP is associated with the translation apparatus. We demonstrate that, in the brain, FMRP is present in actively translating polyribosomes, and we show that this association is acutely sensitive to the type of detergent required to release polyribosomes from membranous structures. In addition, proteomic analyses of purified brain polyribosomes reveal the presence of several RNA-binding proteins that, similarly to FMRP, have been previously localized in neuronal granules. Our findings highlight the complex roles of FMRP both in actively translating polyribosomes and in repressed trafficking ribonucleoparticle granules.
Figures


Similar articles
-
The nuclear microspherule protein 58 is a novel RNA-binding protein that interacts with fragile X mental retardation protein in polyribosomal mRNPs from neurons.Hum Mol Genet. 2006 May 1;15(9):1525-38. doi: 10.1093/hmg/ddl074. Epub 2006 Mar 28. Hum Mol Genet. 2006. PMID: 16571602
-
Tracking the Fragile X Mental Retardation Protein in a Highly Ordered Neuronal RiboNucleoParticles Population: A Link between Stalled Polyribosomes and RNA Granules.PLoS Genet. 2016 Jul 27;12(7):e1006192. doi: 10.1371/journal.pgen.1006192. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27462983 Free PMC article.
-
Fragile X mental retardation: misregulation of protein synthesis in the developing brain?Microsc Res Tech. 2002 May 1;57(3):145-7. doi: 10.1002/jemt.10063. Microsc Res Tech. 2002. PMID: 12112449 Review.
-
Lost once, the Fragile X Mental Retardation protein is now back onto brain polyribosomes.RNA Biol. 2005 Jan;2(1):1-3. doi: 10.4161/rna.2.1.1368. Epub 2005 Jan 15. RNA Biol. 2005. PMID: 17132937
-
mRNPs, polysomes or granules: FMRP in neuronal protein synthesis.Curr Opin Neurobiol. 2006 Jun;16(3):265-9. doi: 10.1016/j.conb.2006.05.010. Epub 2006 May 16. Curr Opin Neurobiol. 2006. PMID: 16707258 Review.
Cited by
-
The unstable repeats--three evolving faces of neurological disease.Neuron. 2013 Mar 6;77(5):825-43. doi: 10.1016/j.neuron.2013.02.022. Neuron. 2013. PMID: 23473314 Free PMC article. Review.
-
Group I metabotropic glutamate receptors: a role in neurodevelopmental disorders?Mol Neurobiol. 2007 Jun;35(3):298-307. doi: 10.1007/s12035-007-0022-1. Mol Neurobiol. 2007. PMID: 17917118 Review.
-
Fragile X Mental Retardation Protein: To Be or Not to Be a Translational Enhancer.Front Mol Biosci. 2018 Dec 11;5:113. doi: 10.3389/fmolb.2018.00113. eCollection 2018. Front Mol Biosci. 2018. PMID: 30619879 Free PMC article. No abstract available.
-
Fragile X syndrome: (What's) lost in translation?Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17329-30. doi: 10.1073/pnas.0408034101. Epub 2004 Dec 6. Proc Natl Acad Sci U S A. 2004. PMID: 15583122 Free PMC article. No abstract available.
-
ncRNA BC1 influences translation in the oocyte.RNA Biol. 2021 Nov;18(11):1893-1904. doi: 10.1080/15476286.2021.1880181. Epub 2021 Feb 8. RNA Biol. 2021. PMID: 33491548 Free PMC article.
References
-
- Darnell, R. B. (2002) Cell 110, 545-550. - PubMed
-
- Dever, T. E. (2002) Cell 108, 545-556. - PubMed
-
- Dreyfuss, G., Kim, V. N. & Kataoka, N. (2002) Nat. Rev. Mol. Cell Biol. 3, 195-205. - PubMed
-
- Kuhl, D. & Skehel, P. (1998) Curr. Opin. Neurobiol. 8, 600-606. - PubMed
-
- Kiebler, M. A. & DesGroseillers, L. (2000) Neuron 25, 19-28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

