Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation
- PMID: 15331397
- PMCID: PMC1618592
- DOI: 10.1016/s0002-9440(10)63335-4
Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation
Abstract
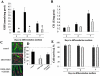
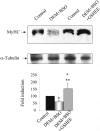
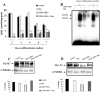
Skeletal muscle differentation is a complex process regulated at multiple levels. This study addressed the effect of glutathione (GSH) depletion on the transition of murine skeletal muscle C2C12 myoblasts into myocytes induced by growth factor inactivation. Cellular GSH levels increased within 24 hours on myogenic stimulation of myoblasts due to enhanced GSH synthetic rate accounted for by stimulated glutamate-L-cysteine ligase (also known as gamma-glutamylcysteine synthetase) activity. In contrast, the synthesis rate of GSH using gamma-glutamylcysteine and glutamate as precursors, which reflects the activity of the GSH synthetase, did not change during differentiation. The stimulation of GSH stores preceded the myogenic differentiation of C2C12 myoblasts monitored by expression of muscle-specific genes, creatine kinase (CK), myosin heavy chain (MyHC), and MyoD. The pattern of DNA binding activity of NF-kappaB and AP-1 in differentiating cells was similar both displaying an activation peak at 24 hours after myogenic stimulation. Depletion of cellular GSH levels 24 hours after stimulation of differentiation abrogated myogenesis as reflected by lower CK activity, MyHC levels, MyoD expression, and myotubes formation, effects that were reversible on GSH replenishment by GSH ethyl ester (GHSEE). Moreover, GSH depletion led to sustained activation of NF-kappaB, while GSHEE prevented it. Furthermore, inhibition of NF-kappaB activation restored myogenesis despite GSH depletion. Thus, GSH contributes to the formation of myotubes from satellite myoblasts by ensuring inactivation of NF-kappaB, and hence maintaining optimal GSH levels may be beneficial in restoring muscle mass in chronic inflammatory disorders.
Figures
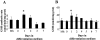
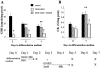

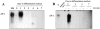

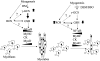
Similar articles
-
S100B protein in myoblasts modulates myogenic differentiation via NF-kappaB-dependent inhibition of MyoD expression.J Cell Physiol. 2010 Apr;223(1):270-82. doi: 10.1002/jcp.22035. J Cell Physiol. 2010. PMID: 20069545
-
Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization.FASEB J. 2004 Feb;18(2):227-37. doi: 10.1096/fj.03-0251com. FASEB J. 2004. PMID: 14769817
-
Inhibition of mechanosensitive cation channels inhibits myogenic differentiation by suppressing the expression of myogenic regulatory factors and caspase-3 activity.FASEB J. 2005 Dec;19(14):1986-97. doi: 10.1096/fj.05-4198com. FASEB J. 2005. PMID: 16319142
-
Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches.Free Radic Biol Med. 2000 May 1;28(9):1405-20. doi: 10.1016/s0891-5849(00)00215-x. Free Radic Biol Med. 2000. PMID: 10924859 Review.
-
Effects of Creatine in Skeletal Muscle Cells and in Myoblasts Differentiating Under Normal or Oxidatively Stressing Conditions.Mini Rev Med Chem. 2016;16(1):4-11. doi: 10.2174/1389557515666150722102342. Mini Rev Med Chem. 2016. PMID: 26202198 Review.
Cited by
-
Age-related changes in skeletal muscle: changes to life-style as a therapy.Biogerontology. 2018 Dec;19(6):519-536. doi: 10.1007/s10522-018-9775-3. Epub 2018 Sep 27. Biogerontology. 2018. PMID: 30259289 Free PMC article. Review.
-
The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis.Cell Death Dis. 2016 Mar 31;7(3):e2168. doi: 10.1038/cddis.2016.50. Cell Death Dis. 2016. PMID: 27031965 Free PMC article.
-
Prostaglandins in muscle regeneration.J Muscle Res Cell Motil. 2008;29(6-8):163-7. doi: 10.1007/s10974-008-9154-9. Epub 2008 Dec 4. J Muscle Res Cell Motil. 2008. PMID: 19052883 Review.
-
SOD1 in ALS: Taking Stock in Pathogenic Mechanisms and the Role of Glial and Muscle Cells.Antioxidants (Basel). 2022 Mar 23;11(4):614. doi: 10.3390/antiox11040614. Antioxidants (Basel). 2022. PMID: 35453299 Free PMC article. Review.
-
Transient glutathione depletion determines terminal differentiation in HL-60 cells.Oxid Med Cell Longev. 2010 Jan-Feb;3(1):53-60. doi: 10.4161/oxim.3.1.10405. Oxid Med Cell Longev. 2010. PMID: 20716928 Free PMC article.
References
-
- Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. - PubMed
-
- Argiles JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999;19:223–248. - PubMed
-
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. - PubMed
-
- Zhao SP, Zeng LH. Elevated plasma levels of tumor necrosis factor in chronic heart failure with cachexia. Int J Cardiol. 1997;58:257–261. - PubMed
-
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-α levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

