Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice
- PMID: 15331409
- PMCID: PMC1618588
- DOI: 10.1016/S0002-9440(10)63347-0
Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice
Abstract
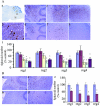
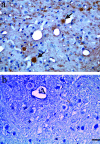
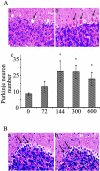
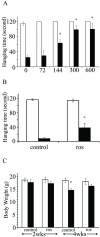
Dysregulation of cyclin-dependent kinases (cdks) and cytoskeletal protein hyperphosphorylation characterizes a subset of human neurodegenerative diseases, including Alzheimer's disease, amyotrophic lateral sclerosis, and Niemann-Pick Type C (NPC). It is thought that these cytoskeletal changes lead eventually to development of hallmark cytoskeletal lesions such as neurofibrillary tangles and axonal spheroids. Although many studies support an involvement of cdks in these neurodegenerative cascades, it is not known whether cdk activity is essential. The naturally occurring npc-1 mutant mouse mimics human NPC, in displaying activation of cdk5, mitotic cdc2, and cdk4, with concomitant cytoskeletal pathology and neurodegeneration. We availed of this model and specific pharmacological inhibitors of cdk activity, to determine whether cdks are necessary for NPC neuropathology. The inhibitors were infused intracerebroventricularly for a 2-week period, initiated at a pathologically incipient stage. While an inactive stereoisomer, iso-olomoucine, was ineffective, two potent inhibitors, roscovitine and olomoucine, attenuated significantly the hyperphosphorylation of neurofilament, tau, and mitotic proteins, reduced the number of spheroids, modulated Purkinje neuron death, and ameliorated motor defects in npc mice. These results suggest that cdk activity is required for neuropathology and subsequent motor impairment in NPC. Studies aimed at knocking down individual cdks in these mice will help identify the specific cdk(s) that are essential, and delineate their precise roles in the neurodegenerative process.
Figures



Similar articles
-
Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model.J Neurosci. 2002 Aug 1;22(15):6515-25. doi: 10.1523/JNEUROSCI.22-15-06515.2002. J Neurosci. 2002. PMID: 12151531 Free PMC article.
-
p35/p25 is not essential for tau and cytoskeletal pathology or neuronal loss in Niemann-Pick type C disease.J Neurosci. 2006 Mar 8;26(10):2738-44. doi: 10.1523/JNEUROSCI.4834-05.2006. J Neurosci. 2006. PMID: 16525053 Free PMC article.
-
Aberrant activation of Cdc2/cyclin B1 is involved in initiation of cytoskeletal pathology in murine Niemann-Pick disease type C.J Huazhong Univ Sci Technolog Med Sci. 2017 Oct;37(5):732-739. doi: 10.1007/s11596-017-1796-7. Epub 2017 Oct 20. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 29058287
-
Cdk5 as a drug target for the treatment of Alzheimer's disease.J Mol Neurosci. 2002 Dec;19(3):267-73. doi: 10.1385/JMN:19:3:267. J Mol Neurosci. 2002. PMID: 12540052 Review.
-
Consequences of NPC1 and NPC2 loss of function in mammalian neurons.Biochim Biophys Acta. 2004 Oct 11;1685(1-3):48-62. doi: 10.1016/j.bbalip.2004.08.011. Biochim Biophys Acta. 2004. PMID: 15465426 Review.
Cited by
-
Long- and short-term CDK5 knockdown prevents spatial memory dysfunction and tau pathology of triple transgenic Alzheimer's mice.Front Aging Neurosci. 2014 Sep 10;6:243. doi: 10.3389/fnagi.2014.00243. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25309427 Free PMC article.
-
Cell cycle molecules define a pathway required for neuron death in development and disease.Biochim Biophys Acta. 2007 Apr;1772(4):392-401. doi: 10.1016/j.bbadis.2006.12.003. Epub 2006 Dec 13. Biochim Biophys Acta. 2007. PMID: 17229557 Free PMC article. Review.
-
Severe neurometabolic phenotype in npc1 -/- zebrafish with a C-terminal mutation.Front Mol Neurosci. 2023 Mar 17;16:1078634. doi: 10.3389/fnmol.2023.1078634. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37008782 Free PMC article.
-
Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease.Curr Med Chem. 2008;15(23):2321-8. doi: 10.2174/092986708785909111. Curr Med Chem. 2008. PMID: 18855662 Free PMC article. Review.
-
Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease.J Neurosci. 2005 Sep 28;25(39):8843-53. doi: 10.1523/JNEUROSCI.2868-05.2005. J Neurosci. 2005. PMID: 16192374 Free PMC article.
References
-
- Goldman JE, Yen SH. Cytoskeletal protein abnormalities in neurodegenerative diseases. Ann Neurol. 1986;19:209–223. - PubMed
-
- Trojanowski JQ, Lee VM. Phosphorylation of neuronal cytoskeletal proteins in Alzheimer’s disease and Lewy body dementias. Ann NY Acad Sci. 1994;747:92–109. - PubMed
-
- Goedert M. Neurofibrillary pathology of Alzheimer’s disease and other tauopathies. Prog Brain Res. 1998;117:287–306. - PubMed
-
- Elleder M, Jirasek A. Neuropathology of various types of Niemann-Pick disease. Acta Neuropathol Suppl (Berl) 1981;7:201–203. - PubMed
-
- Iqbal K, Grundke-Iqbal I. Molecular mechanism of Alzheimer’s neurofibrillary degeneration and therapeutic intervention. Ann NY Acad Sci. 1996;777:132–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

