Angiopoietin-1 causes reversible degradation of the portal microcirculation in mice: implications for treatment of liver disease
- PMID: 15331413
- PMCID: PMC1618608
- DOI: 10.1016/S0002-9440(10)63351-2
Angiopoietin-1 causes reversible degradation of the portal microcirculation in mice: implications for treatment of liver disease
Abstract
In many different liver diseases, such as cirrhosis, degradation of the microcirculation, including obliteration of small portal or hepatic veins contributes to disease-associated portal hypertension. The present study demonstrates the importance of angiogenesis in the establishment of arteriovenous shunts and the accompanying changes to the venous bed. One aspect of angiogenesis involves the branching of new vessels from pre-existing ones, and the molecular mechanisms controlling it are complex and involve a coordinated effort between specific endothelial growth factors and their receptors, including the angiopoietins. We modulated the hepatic vasculature in mice by conditionally expressing angiopoietin-1 in hepatocytes. In mice exposed to angiopoietin-1 during development, arterial sprouting, enlarged arteries, marked loss of portal vein radicles, hepatic vein dilation, and suggestion of arteriovenous shunting were observed. Most importantly, these phenotypic changes were completely reversed within 14 days of turning off transgene expression. Expression of excess angiopoietin-1 beginning in adulthood did not fully recapitulate the phenotype, but did result in enlarged vessels. Our findings suggest that controlling excessive angiogenesis during liver disease may promote the restoration of the portal vein circuit and aid in the resolution of disease-associated portal hypertension.
Figures


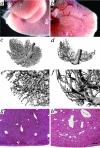


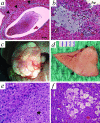


References
-
- Huet PM, Villeneuve JP, Pomier-Layrargues G, Marleau D. Hepatic circulation in cirrhosis. Clin Gastroenterol. 1985;14:155–168. - PubMed
-
- Wanless IR. Epithelioid hemangioendothelioma, multiple focal nodular hyperplasias, and cavernous hemangiomas of the liver. Arch Pathol Lab Med. 2000;124:1105–1107. - PubMed
-
- Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. - PubMed
-
- Wanless IR. Benign liver tumors. Clin Liver Dis. 2002;6:513–526. - PubMed
-
- Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

