Near completely humanized liver in mice shows human-type metabolic responses to drugs
- PMID: 15331414
- PMCID: PMC1618591
- DOI: 10.1016/S0002-9440(10)63352-4
Near completely humanized liver in mice shows human-type metabolic responses to drugs
Abstract
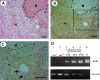
Human hepatocytes were transplanted into urokinase-type plasminogen activator-transgenic SCID mice (uPA/SCID mice), which are immunodeficient and undergo liver failure. The transplanted cells were characterized in terms of their in vivo growth potential and functions. The human hepatocytes progressively repopulated the murine host liver. However, the recipients died when the replacement index (RI) of the human hepatocytes exceeded 50%. The hosts (chimeric mice) survived at RI >50% when treated with a drug that has anti-human complement factor activity, and these mice developed livers with RI values as high as 96%. In total, 36 chimeric mice were generated, and the rate of successful engraftment was as high as 92%. The yield of chimeric mice with RI >70% was 32%. The human hepatocytes in the murine host liver expressed mRNAs for a variety of human cytochrome P450 (hCYP) subtypes, in a manner that was similar to the donor liver. The mRNAs for hCYP3A4 and hCYP1A1/2 were induced in the liver in a CYP type-specific manner when the mice were treated with rifampicin and 3-methylcholanthrene, respectively. These results indicate that human hepatocytes that propagate in mice retain their normal pharmacological responses. We conclude that the chimeric mouse developed in the present study is a useful model for assessing the functions and pharmacological responses of human hepatocytes.
Figures




References
-
- Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. - PubMed
-
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JRT, Tyrrell DLJ, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. - PubMed
-
- Hamatani K, Matsuda Y, Araki R, Itoh M, Abe M. Cloning and chromosomal mapping of the mouse DNA-dependent protein kinase gene. Immunogenetics. 1996;45:1–5. - PubMed
-
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. - PubMed
-
- Hino H, Tateno C, Sato H, Yamasaki C, Katayama S, Kohashi T, Aratani A, Asahara T, Dohi K, Yoshizato K. Long-term culture of human hepatocytes which show a high growth potential and express their differentiated phenotypes. Biochem Biophys Res Commun. 1999;256:184–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

