Morphological characterization of Thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance
- PMID: 15331422
- PMCID: PMC1618604
- DOI: 10.1016/s0002-9440(10)63360-3
Morphological characterization of Thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance
Abstract
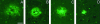
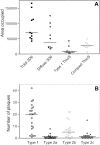
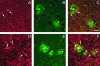
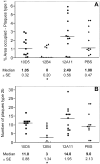
Transgenic mice mimicking certain features of Alzheimer's disease (AD)-pathology, namely amyloid plaques and neurofibrillary tangles, have been developed in an effort to better understand the mechanism leading to the formation of these characteristic cerebral lesions. More recently, these animal models have been widely used to investigate emergent therapies aimed at the reduction of the cerebral amyloid load. Several studies have shown that immunotherapy targeting the amyloid peptide (Abeta) is efficacious at clearing the amyloid plaques or preventing their formation, and at reducing the memory/behavior impairment observed in these animals. In AD, different types of plaques likely have different pathogenic significance, and further characterization of plaque pathology in the PDAPP transgenic mice would enhance the evaluation of potential therapeutics. In the present study, a morphological classification of amyloid plaques present in the brains of PDAPP mice was established by using Thioflavin-S staining. Neuritic dystrophy associated with amyloid plaques was also investigated. Finally, the efficacy of passive immunization with anti-Abeta antibodies on the clearance of Thio-S positive amyloid plaques was studied. Our results show that distinct morphological types of plaques are differentially cleared depending upon the isotype of the antibody.
Figures

References
-
- Mirra SS, Gearing M, Nash F. Neuropathologic assessment of Alzheimer’s disease. Neurology. 1997;49:S14–S16. - PubMed
-
- Delaère P, Duyckaerts C, He Y, Piette F, Hauw JJ. Subtypes and differential laminar distributions of beta A4 deposits in Alzheimer’s disease: relationship with the intellectual status of 26 cases. Acta Neuropathol. 1991;81:328–335. - PubMed
-
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. - PubMed
-
- Knowles RB, Gomez-Isla T, Hyman BT. Abeta associated neuropil changes: correlation with neuronal loss and dementia. J Neuropathol Exp Neurol. 1998;57:1122–1130. - PubMed
-
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

