Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy
- PMID: 15331426
- PMCID: PMC1618594
- DOI: 10.1016/S0002-9440(10)63364-0
Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy
Abstract
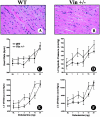
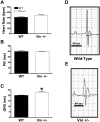
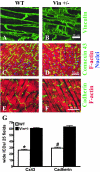
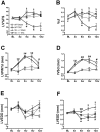
Vinculin and its muscle splice variant metavinculin link focal adhesions and cell-to-cell contact sites to the actin cytoskeleton. We hypothesized that normal expression of vinculin isoforms would be essential for integrity of cardiomyocytes and preservation of normal cardiac function. We studied heterozygous vinculin knockout mice (Vin+/-) that develop and breed normally. The Vin+/- mice displayed: 1) a 58% reduction of vinculin and a 63% reduction of metavinculin protein levels versus wild-type littermates; 2) normal basal cardiac function and histology but abnormal electrocardiograms, intercalated disks, and ICD-related protein distribution; 3) increased mortality following acute hemodynamic stress imposed by transverse aortic constriction (TAC); 4) cardiac dysfunction by 6 weeks post-TAC; and 5) misalignment of alpha-actinin containing Z-lines and abnormal myocardial ultrastructure despite preserved cardiac function. Decreased expression of vinculin/metavinculin leads to abnormal myocyte structure without baseline physiological evidence of cardiac dysfunction. These structural changes predispose to stress-induced cardiomyopathy.
Figures




References
-
- Rudiger M, Korneeva N, Schwienbacher C, Weiss EE, Jockusch BM. Differential actin organization by vinculin isoforms: implications for cell type-specific microfilament anchorage. FEBS Lett. 1998;431:49–54. - PubMed
-
- Schlaepfer DD, Hunter T. Signal transduction from the extracellular matrix—a role for the focal adhesion protein-tyrosine kinase FAK. Cell Struct Funct. 1996;21:445–450. - PubMed
-
- Jockusch BM, Isenberg G. Vinculin and α-actinin: interaction with actin and effect on microfilament network formation. Cold Spring Harbor Symp Quant Biol. 1982;46 Pt 2:613–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

