Membrane bistability in thalamic reticular neurons during spindle oscillations
- PMID: 15331618
- PMCID: PMC2915789
- DOI: 10.1152/jn.00552.2004
Membrane bistability in thalamic reticular neurons during spindle oscillations
Abstract
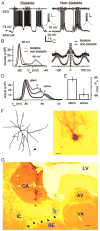
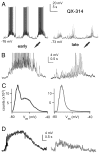
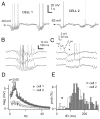
The thalamic reticular (RE) nucleus is a major source of inhibition in the thalamus. It plays a crucial role in regulating the excitability of thalamocortical networks and in generating some sleep rhythms. Current-clamp intracellular recordings of RE neurons in cats under barbiturate anesthesia revealed the presence of membrane bistability in approximately 20% of neurons. Bistability consisted of two alternate membrane potentials, separated by approximately 17-20 mV. While non-bistable (common) RE neurons fired rhythmic spike-bursts during spindles, bistable RE neurons fired tonically, with burst modulation, throughout spindle sequences. Bistability was strongly voltage dependent and only expressed under resting conditions (i.e. no current injection). The transition from the silent to the active state was a regenerative event that could be activated by brief depolarization, whereas brief hyperpolarizations could switch the membrane potential from the active to the silent state. These effects outlasted the current pulses. Corticothalamic stimulation could also switch the membrane potential from silent to active states. Addition of QX-314 in the recording micropipette either abolished or disrupted membrane bistability, suggesting I(Na(p)) to be responsible for its generation. Thalamocortical cells presented various patterns of spindling that reflected the membrane bistability in RE neurons. Finally, experimental data and computer simulations predicted a role for RE neurons' membrane bistability in inducing various patterns of spindling in target thalamocortical cells. We conclude that membrane bistability of RE neurons is an intrinsic property, likely generated by I(Na(p)) and modulated by cortical influences, as well as a factor that determines different patterns of spindle rhythms in thalamocortical neurons.
Figures







References
-
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Cellular and network models for intrathalamic augmenting responses during 10-Hz stimulation. J Neurophysiol. 1998;79:2730–2748. - PubMed
-
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Patterns of spiking-bursting activity in the thalamic reticular nucleus initiate sequences of spindle oscillations in thalamic network. J Neurophysiol. 2000;84:1076–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

