Quantal release of ATP from clusters of PC12 cells
- PMID: 15331685
- PMCID: PMC1665262
- DOI: 10.1113/jphysiol.2004.068924
Quantal release of ATP from clusters of PC12 cells
Abstract
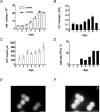
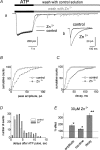
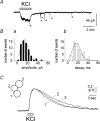
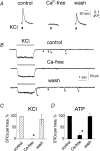
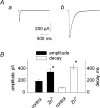
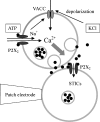
Although ATP is important for intercellular communication, little is known about the mechanism of endogenous ATP release due to a dearth of suitable models. Using PC12 cells known to express the P2X2 subtype of ATP receptors and to store ATP with catecholamines inside dense-core vesicles, we found that clusters of PC12 cells cultured for 3-7 days generated small transient inward currents (STICs) after an inward current elicited by exogenous ATP. The amplitude of STICs in individual cells correlated with the peak amplitude of ATP-induced currents. STICs appeared as asynchronous responses (approximately 20 pA average amplitude) for 1-20 s and were investigated with a combination of patch clamping, Ca2+ imaging, biochemistry and electron microscopy. Comparable STICs were produced by focal KCl pulses and were dependent on extracellular Ca2+. STICs were abolished by the P2X antagonist PPADS and potentiated by Zn2+, suggesting they were mediated by P2X2 receptor activation. The highest probability of observing STICs was after the peak of intracellular Ca2+ increase caused by KCl. Biochemical measurements indicated that KCl application induced a significant release of ATP from PC12 cells. Electron microscopy studies showed narrow clefts without 'synaptic-like' densities between clustered cells. Our data suggest that STICs were caused by quantal release of endogenous ATP by depolarized PC12 cells in close juxtaposition to the recorded cell. Thus, STICs may be a new experimental model to characterize the physiology of vesicular release of ATP and to study the kinetics and pharmacology of P2X2 receptor-mediated quantal currents.
Figures



References
-
- Afework M, Burnstock G. Age-related changes in the localization of P2X (nucleotide) receptors in the rat adrenal gland. Int J Dev Neurosci. 2000;18:515–520. - PubMed
-
- Arslan G, Filipeanu CM, Irenius E, Kull B, Clementi E, Allgaier C, Erlinge D, Fredholm BB. P2Y receptors contribute to ATP-induced increases in intracellular calcium in differentiated but not undifferentiated PC12 cells. Neuropharmacology. 2000;39:482–496. - PubMed
-
- Augustine GJ. How does calcium trigger neurotransmitter release. Curr Opin Neurobiol. 2001;11:320–326. - PubMed
-
- Baldwin SP, Krewson CE, Saltzman WM. PC12 cell aggregation and neurite growth in gels of collagen, laminin and fibronectin. Int J Dev Neurosci. 1996;14:351–364. - PubMed
-
- Baldwin SP, Saltzman WM. Aggregation enhances catecholamine secretion in cultured cells. Tissue Eng. 2001;7:179–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

