Capsid region involved in hepatitis A virus binding to glycophorin A of the erythrocyte membrane
- PMID: 15331714
- PMCID: PMC514964
- DOI: 10.1128/JVI.78.18.9807-9813.2004
Capsid region involved in hepatitis A virus binding to glycophorin A of the erythrocyte membrane
Abstract
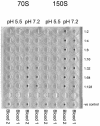
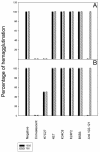
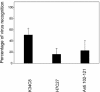
Hepatitis A virus (HAV) has previously been reported to agglutinate human red blood cells at acidic pHs. Treatment of erythrocytes with different enzymes and chemical reagents indicated that HAV attachment is mediated through an interaction with sialylglycoproteins. HAV hemagglutination could be blocked by incubating the virus with glycophorin A, indicating that this sialylglycoprotein is the erythrocyte receptor. The number of receptors used was estimated to be around 500 per cell. At the same time, HAV-induced hemagglutination could also be blocked by either monoclonal antibody H7C27 or an anti-VP3(102-121) ascitic fluid, indicating that lysine 221 of VP1 and the surrounding VP3 residues lining the capsid pit are involved in HAV binding to erythrocytes.
Copyright 2004 American Society for Microbiology
Figures
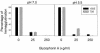
References
-
- Ashida, M., and C. Hamada. 1997. Molecular cloning of the hepatitis A virus receptor from a simian cell line. J. Gen. Virol. 78:1565-1569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

