Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores
- PMID: 15331749
- PMCID: PMC514989
- DOI: 10.1128/JVI.78.18.10166-10177.2004
Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores
Abstract
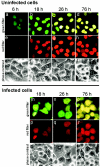
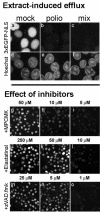
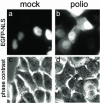
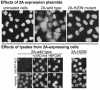
Poliovirus and some other picornaviruses trigger relocation of certain nuclear proteins into the cytoplasm. Here, by using a protein changing its fluorescence color with time and containing a nuclear localization signal (NLS), we demonstrate that the poliovirus-triggered relocation is largely due to the exit of presynthesized nuclear protein into the cytoplasm. The leakiness of the nuclear envelope was also documented by the inability of nuclei from digitonin-permeabilized, virus-infected (but not mock-infected) cells to retain an NLS-containing derivative of green fluorescent protein (GFP). The cytoplasm-to-nucleus traffic was also facilitated during infection, as evidenced by experiments with GAPDH (glyceraldehyde-3-phosphate dehydrogenase), cyclin B1, and an NLS-lacking derivative of GFP, which are predominantly cytoplasmic in uninfected cells. Electron microscopy demonstrated that a bar-like barrier structure in the channel of the nuclear pores, seen in uninfected cells, was missing in the infected cells, giving the impression of fully open pores. Transient expression of poliovirus 2A protease also resulted in relocation of the nuclear proteins. Lysates from poliovirus-infected or 2A-expressing cells induced efflux of 3xEGFP-NLS from the nuclei of permeabilized uninfected cells. This activity was inhibited by the elastase inhibitors elastatinal and N-(methoxysuccinyl)-L-alanyl-L-alanyl-L-prolyl-L-valine chloromethylketone (drugs known also to be inhibitors of poliovirus protease 2A), a caspase inhibitor zVAD(OMe), fmk, and some other protease inhibitors. These data suggest that 2A elicited nuclear efflux, possibly in cooperation with a zVAD(OMe).fmk-sensitive protease. However, poliovirus infection facilitated nuclear protein efflux also in cells deficient in caspase-3 and caspase-9, suggesting that the efflux may occur without the involvement of these enzymes. The biological relevance of nucleocytoplasmic traffic alterations in infected cells is discussed.
Copyright 2004 American Society for Microbiology
Figures




References
-
- Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T., Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:342-353. - PubMed
-
- Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis viral protein 2A localizes to nucleoli and inhibits cap-dependent mRNA translation. Virus Res. 95:45-57. - PubMed
-
- Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 95:59-73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

