Anticorrelated motions as a driving force in enzyme catalysis: the dehydrogenase reaction
- PMID: 15331786
- PMCID: PMC516540
- DOI: 10.1073/pnas.0405502101
Anticorrelated motions as a driving force in enzyme catalysis: the dehydrogenase reaction
Abstract
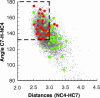
Molecular dynamics and cross-correlation analysis of the horse liver alcohol dehydrogenase HLADH.NAD(+).PhCH(2)O(-) complex has established anticorrelated motions between the NAD(+)-binding domain and other portions of the enzyme. Four pairs of anticorrelated interactions are (i and ii) cofactor-binding domain: C(alpha) of V292 and the CG1 of V203 with C7 of PhCH(2)O(-); (iii) cofactor-binding domain: amide carbonyl oxygen of I318 with amide N of H67; and (iv) cofactor domain: C(alpha) of T178 with carbonyl oxygen of L141. The average distances between pairs are 9.2 A for i, 8.2 A for ii, 14.7 A for iii, and 18.2 A for iv. The motions of i and ii are most important in the approximately 0.5 A pushing of C4 of NAD(+) toward C7 of PhCH(2)O(-) to form push near-attack conformer (NACs). The motions of iv are less so, and those of iii are not important. Seventy-five quantum mechanics/molecular mechanics calculations of the energies of reaction were carried out without structural restrictions from different stages of the molecular dynamics trajectory. Of the 71 conformations, the 29 fulfilling NAC criteria were associated with the lowest energies of activation. Thus, anticorrelated motions from the NAD(+)-binding domain by way of the neighboring V292 and V203 have a pushing motion, which moves the C4 of NAD(+) toward the H-C7 of the substrate. Longer-range anticorrelated motions involving the cofactor-binding domain have no or very little influence on NAC formation.
Figures


References
-
- Mincer, J. S. & Schwartz, S. D. (2003) J. Proteome Res. 2, 437–439. - PubMed
-
- Caratzoulas, S., Mincer, J. S. & Schwartz, S. D. (2002) J. Am. Chem. Soc. 124, 3270–3276. - PubMed
-
- Mincer, J. S. & Schwartz, S. D. (2003) J. Phys. Chem. B 107, 366–371.
-
- Benkovic, S. J. & Hammes-Schiffer, S. (2003) Science 301, 1196–1202. - PubMed
-
- Bahnson, B. J., Park, D. H., Kim, K., Plapp, B. V. & Klinman, J. P. (1993) Biochemistry 32, 5503–5507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

