Incorporation of a single His residue by rational design enables thiol-ester hydrolysis by human glutathione transferase A1-1
- PMID: 15333749
- PMCID: PMC516542
- DOI: 10.1073/pnas.0403045101
Incorporation of a single His residue by rational design enables thiol-ester hydrolysis by human glutathione transferase A1-1
Abstract
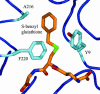
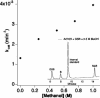
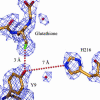
A strategy for rational enzyme design is reported and illustrated by the engineering of a protein catalyst for thiol-ester hydrolysis. Five mutants of human glutathione (GSH; gamma-Glu-Cys-Gly) transferase A1-1 were designed in the search for a catalyst and to provide a set of proteins from which the reaction mechanism could be elucidated. The single mutant A216H catalyzed the hydrolysis of the S-benzoyl ester of GSH under turnover conditions with a k(cat)/K(M) of 156 M(-1) x min(-1), and a catalytic proficiency of >10(7) M(-1) when compared with the first-order rate constant of the uncatalyzed reaction. The wild-type enzyme did not hydrolyze the substrate, and thus, the introduction of a single histidine residue transformed the wild-type enzyme into a turnover system for thiol-ester hydrolysis. By kinetic analysis of single, double, and triple mutants, as well as from studies of reaction products, it was established that the enzyme A216H catalyzes the hydrolysis of the thiol-ester substrate by a mechanism that includes an acyl intermediate at the side chain of Y9. Kinetic measurements and the crystal structure of the A216H GSH complex provided compelling evidence that H216 acts as a general-base catalyst. The introduction of a single His residue into human GSH transferase A1-1 created an unprecedented enzymatic function, suggesting a strategy that may be of broad applicability in the design of new enzymes. The protein catalyst has the hallmarks of a native enzyme and is expected to catalyze various hydrolytic, as well as transesterification, reactions.
Figures


References
-
- Cedrone, F., Menez, A. & Quemeneur, E. (2000) Curr. Opin. Struct. Biol. 10, 405–410. - PubMed
-
- Penning, T. M. & Jez, J. M. (2001) Chem. Rev. 101, 3027–3046. - PubMed
-
- Qi, D. F., Tann, C. M., Haring, D. & Distefano, M. D. (2001) Chem. Rev. 101, 3081–3111. - PubMed
-
- Jez, J. M. & Penning, T. M. (1998) Biochemistry 37, 9695–9703. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

