Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management
- PMID: 15337730
- PMCID: PMC514646
- DOI: 10.1503/cmaj.1031698
Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management
Abstract
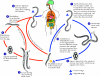
Strongyloidiasis, which is caused by the nematode Strongyloides stercoralis, is a common and persistent infection, particularly in developing countries. In the setting of compromised cellular immunity, it can result in fulminant dissemination with case-fatality rates of over 70%. The majority of new Canadian immigrants come from countries where Strongyloides is highly endemic; therefore, the burden of Strongyloides may be underappreciated in Canada. Because early diagnosis and therapy can have a marked impact on disease outcome, screening for this infection should be considered mandatory for patients who have a history of travel or residence in a disease-endemic area and risk factors for disseminated disease (e.g., corticosteroid use and human T-lymphotropic virus type I infection).
Figures


References
-
- Adedayo O, Grell G, Bellot P. Hyperinfective strongyloidiasis in the medical ward: review of 27 cases in 5 years. South Med J 2002;95(7):711-6. - PubMed
-
- Siddiqui A, Berk S. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 2001; 33:1040-7. - PubMed
-
- Statistics Canada. 2001 Census nation tables. Ottawa: Statistics Canada; 2002.
-
- Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis 1989; 11:755-67. - PubMed
-
- Gyorkos TW, Genta RM, Viens P, MacLean JD. Seroepidemiology of Strongyloides infection in the Southeast Asian refugee population in Canada. Am J Epidemiol 1990;132:257-64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
