Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly
- PMID: 15337773
- PMCID: PMC2172416
- DOI: 10.1083/jcb.200405023
Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly
Abstract
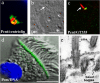
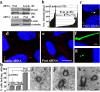
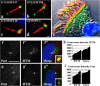
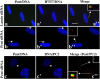
Primary cilia are nonmotile microtubule structures that assemble from basal bodies by a process called intraflagellar transport (IFT) and are associated with several human diseases. Here, we show that the centrosome protein pericentrin (Pcnt) colocalizes with IFT proteins to the base of primary and motile cilia. Immunogold electron microscopy demonstrates that Pcnt is on or near basal bodies at the base of cilia. Pcnt depletion by RNA interference disrupts basal body localization of IFT proteins and the cation channel polycystin-2 (PC2), and inhibits primary cilia assembly in human epithelial cells. Conversely, silencing of IFT20 mislocalizes Pcnt from basal bodies and inhibits primary cilia assembly. Pcnt is found in spermatocyte IFT fractions, and IFT proteins are found in isolated centrosome fractions. Pcnt antibodies coimmunoprecipitate IFT proteins and PC2 from several cell lines and tissues. We conclude that Pcnt, IFTs, and PC2 form a complex in vertebrate cells that is required for assembly of primary cilia and possibly motile cilia and flagella.
Figures

Similar articles
-
The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly.Mol Biol Cell. 2006 Sep;17(9):3781-92. doi: 10.1091/mbc.e06-02-0133. Epub 2006 Jun 14. Mol Biol Cell. 2006. PMID: 16775004 Free PMC article.
-
Evidence for intraflagellar transport in butterfly spermatocyte cilia.Cytoskeleton (Hoboken). 2023 May-Jun;80(5-6):112-122. doi: 10.1002/cm.21755. Epub 2023 Apr 10. Cytoskeleton (Hoboken). 2023. PMID: 37036073 Free PMC article.
-
Pericentrin, a centrosomal protein related to microcephalic primordial dwarfism, is required for olfactory cilia assembly in mice.FASEB J. 2009 Oct;23(10):3289-97. doi: 10.1096/fj.08-124420. Epub 2009 May 26. FASEB J. 2009. PMID: 19470799
-
Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton.Ann N Y Acad Sci. 2015 Jan;1335(1):78-99. doi: 10.1111/nyas.12463. Epub 2014 Jun 24. Ann N Y Acad Sci. 2015. PMID: 24961486 Free PMC article. Review.
-
Role of DZIP1-CBY-FAM92 transition zone complex in the basal body to membrane attachment and ciliary budding.Biochem Soc Trans. 2020 Jun 30;48(3):1067-1075. doi: 10.1042/BST20191007. Biochem Soc Trans. 2020. PMID: 32491167 Review.
Cited by
-
Polycystin-1 regulates ARHGAP35-dependent centrosomal RhoA activation and ROCK signaling.JCI Insight. 2020 Aug 20;5(16):e135385. doi: 10.1172/jci.insight.135385. JCI Insight. 2020. PMID: 32663194 Free PMC article.
-
Deletion of CEP164 in mouse photoreceptors post-ciliogenesis interrupts ciliary intraflagellar transport (IFT).PLoS Genet. 2022 Sep 8;18(9):e1010154. doi: 10.1371/journal.pgen.1010154. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36074756 Free PMC article.
-
Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity.Mol Biol Cell. 2007 Sep;18(9):3667-80. doi: 10.1091/mbc.e06-07-0604. Epub 2007 Jul 11. Mol Biol Cell. 2007. PMID: 17626165 Free PMC article.
-
Rab35 controls cilium length, function and membrane composition.EMBO Rep. 2019 Oct 4;20(10):e47625. doi: 10.15252/embr.201847625. Epub 2019 Aug 21. EMBO Rep. 2019. PMID: 31432619 Free PMC article.
-
Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):E1723-30. doi: 10.1073/pnas.1403470111. Epub 2014 Mar 24. Proc Natl Acad Sci U S A. 2014. PMID: 24706852 Free PMC article.
References
-
- Cole, D.G., D.R. Diener, A.L. Himelblau, P.L. Beech, J.C. Fuster, and J.L. Rosenbaum. 1998. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141:993–1008. - PMC - PubMed
-
- Deane, J.A., D.G. Cole, E.S. Seeley, D.R. Diener, and J.L. Rosenbaum. 2001. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11:1586–1590. - PubMed
-
- Doxsey, S.J. 2001. Centrosomes as command centres for cellular control. Nat. Cell Biol. 3:E105–E108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

