Sorting of a nonmuscle tropomyosin to a novel cytoskeletal compartment in skeletal muscle results in muscular dystrophy
- PMID: 15337777
- PMCID: PMC2172434
- DOI: 10.1083/jcb.200406181
Sorting of a nonmuscle tropomyosin to a novel cytoskeletal compartment in skeletal muscle results in muscular dystrophy
Abstract
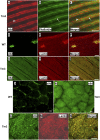
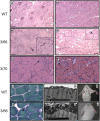
Tropomyosin (Tm) is a key component of the actin cytoskeleton and >40 isoforms have been described in mammals. In addition to the isoforms in the sarcomere, we now report the existence of two nonsarcomeric (NS) isoforms in skeletal muscle. These isoforms are excluded from the thin filament of the sarcomere and are localized to a novel Z-line adjacent structure. Immunostained cross sections indicate that one Tm defines a Z-line adjacent structure common to all myofibers, whereas the second Tm defines a spatially distinct structure unique to muscles that undergo chronic or repetitive contractions. When a Tm (Tm3) that is normally absent from muscle was expressed in mice it became associated with the Z-line adjacent structure. These mice display a muscular dystrophy and ragged-red fiber phenotype, suggestive of disruption of the membrane-associated cytoskeletal network. Our findings raise the possibility that mutations in these tropomyosin and these structures may underpin these types of myopathies.
Figures








Similar articles
-
A cytoskeletal tropomyosin can compromise the structural integrity of skeletal muscle.Cell Motil Cytoskeleton. 2009 Sep;66(9):710-20. doi: 10.1002/cm.20400. Cell Motil Cytoskeleton. 2009. PMID: 19530183
-
Tropomyosin 4 defines novel filaments in skeletal muscle associated with muscle remodelling/regeneration in normal and diseased muscle.Cell Motil Cytoskeleton. 2008 Jan;65(1):73-85. doi: 10.1002/cm.20245. Cell Motil Cytoskeleton. 2008. PMID: 17968984
-
Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity.Circ Res. 2007 Jul 20;101(2):205-14. doi: 10.1161/CIRCRESAHA.107.148379. Epub 2007 Jun 7. Circ Res. 2007. PMID: 17556658
-
Tropomyosin isoforms: divining rods for actin cytoskeleton function.Trends Cell Biol. 2005 Jun;15(6):333-41. doi: 10.1016/j.tcb.2005.04.007. Trends Cell Biol. 2005. PMID: 15953552 Review.
-
Congenital myopathies: diseases of the actin cytoskeleton.J Pathol. 2004 Nov;204(4):407-17. doi: 10.1002/path.1648. J Pathol. 2004. PMID: 15495263 Review.
Cited by
-
Regulation of cell proliferation by ERK and signal-dependent nuclear translocation of ERK is dependent on Tm5NM1-containing actin filaments.Mol Biol Cell. 2015 Jul 1;26(13):2475-90. doi: 10.1091/mbc.E14-10-1453. Epub 2015 May 13. Mol Biol Cell. 2015. PMID: 25971798 Free PMC article.
-
Triadins are not triad-specific proteins: two new skeletal muscle triadins possibly involved in the architecture of sarcoplasmic reticulum.J Biol Chem. 2005 Aug 5;280(31):28601-9. doi: 10.1074/jbc.M501484200. Epub 2005 May 31. J Biol Chem. 2005. PMID: 15927957 Free PMC article.
-
Tropomyosin isoforms and reagents.Bioarchitecture. 2011 Jul;1(4):135-164. doi: 10.4161/bioa.1.4.17897. Epub 2011 Jul 1. Bioarchitecture. 2011. PMID: 22069507 Free PMC article.
-
Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting.Aging (Albany NY). 2012 Feb;4(2):133-43. doi: 10.18632/aging.100436. Aging (Albany NY). 2012. PMID: 22361433 Free PMC article.
-
The sole essential low molecular weight tropomyosin isoform of Caenorhabditis elegans is essential for pharyngeal muscle function.bioRxiv [Preprint]. 2024 Dec 18:2024.12.13.628433. doi: 10.1101/2024.12.13.628433. bioRxiv. 2024. Update in: Cytoskeleton (Hoboken). 2025 Mar 13. doi: 10.1002/cm.22014. PMID: 39764053 Free PMC article. Updated. Preprint.
References
-
- Arai, A., J.A. Spencer, and E.N. Olson. 2002. STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J. Biol. Chem. 277:24453–24459. - PubMed
-
- Banker, B.Q., and A.G. Engel. 1994. Basic Reactions of Muscle. Myology. A.G. Engel and Franzini-Armstrong C., editors. McGraw-Hill, Inc., New York. 832–888.
-
- Bittner, R.E., L.V. Anderson, E. Burkhardt, R. Bashir, E. Vafiadaki, S. Ivanova, T. Raffelsberger, I. Maerk, H. Hoger, M. Jung, et al. 1999. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat. Genet. 23:141–142. - PubMed
-
- Borrione, A.C., A.M. Zanellato, L. Saggin, M. Mazzoli, G. Azzarello, and S. Sartore. 1988. Neonatal myosin heavy chains are not expressed in Ni-induced rat rhabdomyosarcoma. Differentiation. 38:49–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

