The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes
- PMID: 15337789
- PMCID: PMC2212739
- DOI: 10.1084/jem.20040773
The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes
Abstract
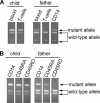
Both innate and adaptive immune responses are dependent on activation of nuclear factor kappaB (NF-kappaB), induced upon binding of pathogen-associated molecular patterns to Toll-like receptors (TLRs). In murine models, defects in NF-kappaB pathway are often lethal and viable knockout mice have severe immune defects. Similarly, defects in the human NF-kappaB pathway described to date lead to severe clinical disease. Here, we describe a patient with a hyper immunoglobulin M-like immunodeficiency syndrome and ectodermal dysplasia. Monocytes did not produce interleukin 12p40 upon stimulation with various TLR stimuli and nuclear translocation of NF-kappaB was impaired. T cell receptor-mediated proliferation was also impaired. A heterozygous mutation was found at serine 32 in IkappaBalpha. Interestingly, his father has the same mutation but displays complex mosaicism. He does not display features of ectodermal dysplasia and did not suffer from serious infections with the exception of a relapsing Salmonella typhimurium infection. His monocyte function was impaired, whereas T cell function was relatively normal. Consistent with this, his T cells almost exclusively displayed the wild-type allele, whereas both alleles were present in his monocytes. We propose that the T and B cell compartment of the mosaic father arose as a result of selection of wild-type cells and that this underlies the widely different clinical phenotype.
Figures



Similar articles
-
Heterozygous N-terminal deletion of IkappaBalpha results in functional nuclear factor kappaB haploinsufficiency, ectodermal dysplasia, and immune deficiency.J Allergy Clin Immunol. 2007 Oct;120(4):900-7. doi: 10.1016/j.jaci.2007.08.035. J Allergy Clin Immunol. 2007. PMID: 17931563
-
A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency.J Clin Invest. 2003 Oct;112(7):1108-15. doi: 10.1172/JCI18714. J Clin Invest. 2003. PMID: 14523047 Free PMC article.
-
Mechanisms of genotype-phenotype correlation in autosomal dominant anhidrotic ectodermal dysplasia with immune deficiency.J Allergy Clin Immunol. 2018 Mar;141(3):1060-1073.e3. doi: 10.1016/j.jaci.2017.05.030. Epub 2017 Jun 17. J Allergy Clin Immunol. 2018. PMID: 28629746 Free PMC article.
-
Human IκBα Gain of Function: a Severe and Syndromic Immunodeficiency.J Clin Immunol. 2017 Jul;37(5):397-412. doi: 10.1007/s10875-017-0400-z. Epub 2017 Jun 9. J Clin Immunol. 2017. PMID: 28597146 Free PMC article. Review.
-
Diagnosis and treatment in anhidrotic ectodermal dysplasia with immunodeficiency.Allergol Int. 2012 Jun;61(2):207-17. doi: 10.2332/allergolint.12-RAI-0446. Allergol Int. 2012. PMID: 22635013 Review.
Cited by
-
Exogenous signal-independent nuclear IkappaB kinase activation triggered by Nkx3.2 enables constitutive nuclear degradation of IkappaB-alpha in chondrocytes.Mol Cell Biol. 2011 Jul;31(14):2802-16. doi: 10.1128/MCB.00253-10. Epub 2011 May 23. Mol Cell Biol. 2011. PMID: 21606193 Free PMC article.
-
Inflammatory Diseases and Growth: Effects on the GH-IGF Axis and on Growth Plate.Int J Mol Sci. 2017 Aug 31;18(9):1878. doi: 10.3390/ijms18091878. Int J Mol Sci. 2017. PMID: 28858208 Free PMC article. Review.
-
Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind.Curr Opin Immunol. 2015 Feb;32:90-105. doi: 10.1016/j.coi.2015.01.005. Epub 2015 Jan 31. Curr Opin Immunol. 2015. PMID: 25645939 Free PMC article. Review.
-
Acute Bacterial Gastroenteritis.Gastroenterol Clin North Am. 2021 Jun;50(2):283-304. doi: 10.1016/j.gtc.2021.02.002. Epub 2021 Apr 23. Gastroenterol Clin North Am. 2021. PMID: 34024442 Free PMC article. Review.
-
Gene hunting in the genomic era: approaches to diagnostic dilemmas in patients with primary immunodeficiencies.J Allergy Clin Immunol. 2014 Aug;134(2):262-8. doi: 10.1016/j.jaci.2013.08.021. Epub 2013 Oct 5. J Allergy Clin Immunol. 2014. PMID: 24100122 Free PMC article. Review.
References
-
- O'Brien, A.D., D.L. Rosenstreich, I. Scher, G.H. Campbell, R.P. MacDermott, and S.B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 124:20–24. - PubMed
-
- Feng, C.G., C.A. Scanga, C.M. Collazo-Custodio, A.W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758–4764. - PubMed
-
- Mun, H.S., F. Aosai, K. Norose, M. Chen, L.X. Piao, O. Takeuchi, S. Akira, H. Ishikura, and A. Yano. 2003. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 15:1081–1087. - PubMed
-
- Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396. - PubMed
-
- Seki, E., H. Tsutsui, N.M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, et al. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863–3868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

