How source content determines intracellular Ca2+ release kinetics. Simultaneous measurement of [Ca2+] transients and [H+] displacement in skeletal muscle
- PMID: 15337820
- PMCID: PMC2233888
- DOI: 10.1085/jgp.200409071
How source content determines intracellular Ca2+ release kinetics. Simultaneous measurement of [Ca2+] transients and [H+] displacement in skeletal muscle
Abstract
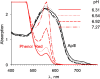
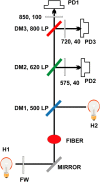
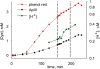
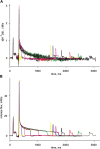
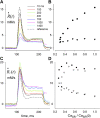
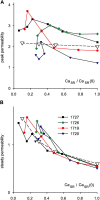
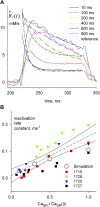
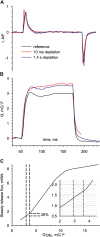
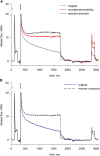
In skeletal muscle, the waveform of Ca(2+) release under clamp depolarization exhibits an early peak. Its decay reflects an inactivation, which locally corresponds to the termination of Ca(2+) sparks, and is crucial for rapid control. In cardiac muscle, both the frequency of spontaneous sparks (i.e., their activation) and their termination appear to be strongly dependent on the Ca(2+) content in the sarcoplasmic reticulum (SR). In skeletal muscle, no such role is established. Seeking a robust measurement of Ca(2+) release and a way to reliably modify the SR content, we combined in the same cells the "EGTA/phenol red" method (Pape et al., 1995) to evaluate Ca(2+) release, with the "removal" method (Melzer et al., 1987) to evaluate release flux. The cytosol of voltage-clamped frog fibers was equilibrated with EGTA (36 mM), antipyrylazo III, and phenol red, and absorbance changes were monitored simultaneously at three wavelengths, affording largely independent evaluations of Delta[H(+)] and Delta[Ca(2+)] from which the amount of released Ca(2+) and the release flux were independently derived. Both methods yielded mutually consistent evaluations of flux. While the removal method gave a better kinetic picture of the release waveform, EGTA/phenol red provided continuous reproducible measures of calcium in the SR (Ca(SR)). Steady release permeability (P), reached at the end of a 120-ms pulse, increased as Ca(SR) was progressively reduced by a prior conditioning pulse, reaching 2.34-fold at 25% of resting Ca(SR) (four cells). Peak P, reached early during a pulse, increased proportionally much less with SR depletion, decreasing at very low Ca(SR). The increase in steady P upon depletion was associated with a slowing of the rate of decay of P after the peak (i.e., a slower inactivation of Ca(2+) release). These results are consistent with a major inhibitory effect of cytosolic (rather than intra-SR) Ca(2+) on the activity of Ca(2+) release channels.
Figures



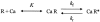

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

