Facilitating arrhythmia simulation: the method of quantitative cellular automata modeling and parallel running
- PMID: 15339335
- PMCID: PMC517726
- DOI: 10.1186/1475-925X-3-29
Facilitating arrhythmia simulation: the method of quantitative cellular automata modeling and parallel running
Abstract
Background: Many arrhythmias are triggered by abnormal electrical activity at the ionic channel and cell level, and then evolve spatio-temporally within the heart. To understand arrhythmias better and to diagnose them more precisely by their ECG waveforms, a whole-heart model is required to explore the association between the massively parallel activities at the channel/cell level and the integrative electrophysiological phenomena at organ level.
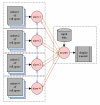
Methods: We have developed a method to build large-scale electrophysiological models by using extended cellular automata, and to run such models on a cluster of shared memory machines. We describe here the method, including the extension of a language-based cellular automaton to implement quantitative computing, the building of a whole-heart model with Visible Human Project data, the parallelization of the model on a cluster of shared memory computers with OpenMP and MPI hybrid programming, and a simulation algorithm that links cellular activity with the ECG.
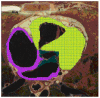
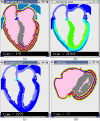
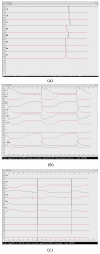
Results: We demonstrate that electrical activities at channel, cell, and organ levels can be traced and captured conveniently in our extended cellular automaton system. Examples of some ECG waveforms simulated with a 2-D slice are given to support the ECG simulation algorithm. A performance evaluation of the 3-D model on a four-node cluster is also given.
Conclusions: Quantitative multicellular modeling with extended cellular automata is a highly efficient and widely applicable method to weave experimental data at different levels into computational models. This process can be used to investigate complex and collective biological activities that can be described neither by their governing differentiation equations nor by discrete parallel computation. Transparent cluster computing is a convenient and effective method to make time-consuming simulation feasible. Arrhythmias, as a typical case, can be effectively simulated with the methods described.
Figures


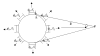
 is the normal direction of the transjunctional potential difference between the current cell and its first neighboring cell; ... Ω is the solid angle subtended by the current cell at field point P.
is the normal direction of the transjunctional potential difference between the current cell and its first neighboring cell; ... Ω is the solid angle subtended by the current cell at field point P.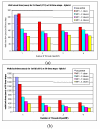
References
-
- Fleischmann PH, Stark G, Wach P. The antiarrhythmic effect of verapamil on atrioventricular re-entry in the Wolff-Parkinson-White syndrome: a computer model study. Int J Biomed Comput. 1996;41:125–136. - PubMed
-
- Gulrajani RM, Savard P, Roberge FA. The inverse problem in electrocardiography: solutions in terms of equivalent sources. Crit Rev Biomed Eng. 1988;16:171–214. - PubMed
-
- Shahidi AV, Savard P. Forward problem of electrocardiography: construction of human torso models and field calculations using finite element method. Med Biol Eng Comput. 1994;32:S25–S33. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

