Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites
- PMID: 15339809
- PMCID: PMC1304787
- DOI: 10.1529/biophysj.104.049833
Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites
Abstract
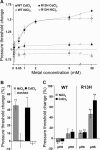
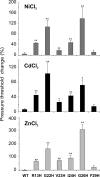
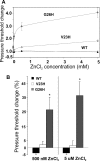
The mechanosensitive channel of large conductance, MscL, of Escherichia coli is one of the best-studied mechanosensitive proteins. Although the structure of the closed or "nearly-closed" state of the Mycobacterium tuberculosis ortholog has been solved and mechanisms of gating have been proposed, the transition from the closed to the open states remains controversial. Here, we probe the relative position of specific residues predicted to line the pore of MscL in either the closed state or during the closed-to-open transition by engineering single-site histidine substitutions and assessing the ability of Ni2+, Cd2+ or Zn2+ ions to affect channel activity. All residues predicted to be within the pore led to a change in channel threshold pressure, although the direction and extent of this change were dependent upon the mutation and metal used. One of the MscL mutants, L19H, exhibited gating that was inhibited by Cd2+ but stimulated by Ni2+, suggesting that these metals bind to and influence different states of the channel. Together, the results derived from this study support the hypotheses that the crystal structure depicts a "nearly closed" rather than a "fully closed" state of MscL, and that a clockwise rotation of transmembrane domain 1 occurs early in the gating process.
Figures



Similar articles
-
The mechanosensitive channel of small conductance (MscS) functions as a Jack-in-the box.Biochim Biophys Acta. 2015 Jan;1848(1 Pt A):159-66. doi: 10.1016/j.bbamem.2014.10.022. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25450806 Free PMC article.
-
Novel compounds that specifically bind and modulate MscL: insights into channel gating mechanisms.FASEB J. 2019 Mar;33(3):3180-3189. doi: 10.1096/fj.201801628R. Epub 2018 Oct 25. FASEB J. 2019. PMID: 30359098 Free PMC article.
-
An in vivo assay identifies changes in residue accessibility on mechanosensitive channel gating.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10161-5. doi: 10.1073/pnas.0402040101. Epub 2004 Jun 28. Proc Natl Acad Sci U S A. 2004. PMID: 15226501 Free PMC article.
-
Gating the bacterial mechanosensitive channels: MscS a new paradigm?Curr Opin Microbiol. 2004 Apr;7(2):163-7. doi: 10.1016/j.mib.2004.02.006. Curr Opin Microbiol. 2004. PMID: 15063854 Review.
-
MscL: channeling membrane tension.Pflugers Arch. 2015 Jan;467(1):15-25. doi: 10.1007/s00424-014-1535-x. Epub 2014 May 27. Pflugers Arch. 2015. PMID: 24859800 Free PMC article. Review.
Cited by
-
The dynamics of protein-protein interactions between domains of MscL at the cytoplasmic-lipid interface.Channels (Austin). 2012 Jul-Aug;6(4):255-61. doi: 10.4161/chan.20756. Epub 2012 Jul 1. Channels (Austin). 2012. PMID: 22874845 Free PMC article.
-
Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target.Microbiol Mol Biol Rev. 2020 Jan 15;84(1):e00055-19. doi: 10.1128/MMBR.00055-19. Print 2020 Feb 19. Microbiol Mol Biol Rev. 2020. PMID: 31941768 Free PMC article. Review.
-
Gating of the mechanosensitive channel protein MscL: the interplay of membrane and protein.Biophys J. 2008 May 1;94(9):3497-511. doi: 10.1529/biophysj.107.109850. Epub 2008 Jan 22. Biophys J. 2008. PMID: 18212020 Free PMC article.
-
Scanning MscL Channels with Targeted Post-Translational Modifications for Functional Alterations.PLoS One. 2015 Sep 14;10(9):e0137994. doi: 10.1371/journal.pone.0137994. eCollection 2015. PLoS One. 2015. PMID: 26368283 Free PMC article.
-
An open-pore structure of the mechanosensitive channel MscL derived by determining transmembrane domain interactions upon gating.FASEB J. 2009 Jul;23(7):2197-204. doi: 10.1096/fj.09-129296. Epub 2009 Mar 4. FASEB J. 2009. PMID: 19261722 Free PMC article.
References
-
- Berrier, C., M. Besnard, B. Ajouz, A. Coulombe, and A. Ghazi. 1996. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol. 151:175–187. - PubMed
-
- Betanzos, M., C. S. Chiang, H. R. Guy, and S. Sukharev. 2002. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat. Struct. Biol. 9:704–710. - PubMed
-
- Blount, P., and P. Moe. 1999. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 7:420–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

