The protonation status of compound II in myoglobin, studied by a combination of experimental data and quantum chemical calculations: quantum refinement
- PMID: 15339813
- PMCID: PMC1304810
- DOI: 10.1529/biophysj.104.041590
The protonation status of compound II in myoglobin, studied by a combination of experimental data and quantum chemical calculations: quantum refinement
Abstract
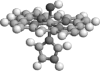
Treatment of met-myoglobin (FeIII) with H2O2 gives rise to ferryl myoglobin, which is closely related to compound II in peroxidases. Experimental studies have given conflicting results for this species. In particular, crystallographic and extended x-ray absorption fine-structure data have shown either a short (approximately 170 pm) or a longer (approximately 190 pm) Fe-O bond, indicating either a double or a single bond. We here present a combined experimental and theoretical investigation of this species. In particular, we use quantum refinement to re-refine a crystal structure with a long bond, using 12 possible states of the active site. The states differ in the formal oxidation state of the iron ion and in the protonation of the oxygen ligand (O2-, OH-, or H2O) and the distal histidine residue (with a proton on Ndelta1, Nepsilon2, or on both atoms). Quantum refinement is essentially standard crystallographic refinement, where the molecular-mechanics potential, normally used to supplement the experimental data, is replaced by a quantum chemical calculation. Thereby, we obtain an accurate description of the active site in all the different protonation and oxidation states, and we can determine which of the 12 structures fit the experimental data best by comparing the crystallographic R-factors, electron-density maps, strain energies, and deviation from the ideal structure. The results indicate that FeIII OH- and FeIV OH- fit the experimental data almost equally well. These two states are appreciably better than the standard model of compound II, FeIV O2-. Combined with the available spectroscopic data, this indicates that compound II in myoglobin is protonated and is best described as FeIV OH-. It accepts a hydrogen bond from the distal His, which may be protonated at low pH.
Figures



Similar articles
-
Crystallographic and spectroscopic studies of peroxide-derived myoglobin compound II and occurrence of protonated FeIV O.J Biol Chem. 2007 Aug 10;282(32):23372-86. doi: 10.1074/jbc.M701948200. Epub 2007 Jun 12. J Biol Chem. 2007. PMID: 17565988
-
Theoretical study of the discrimination between O(2) and CO by myoglobin.J Inorg Biochem. 2002 Jul 25;91(1):101-15. doi: 10.1016/s0162-0134(02)00426-9. J Inorg Biochem. 2002. PMID: 12121767
-
EPR and ENDOR studies of cryoreduced compounds II of peroxidases and myoglobin. Proton-coupled electron transfer and protonation status of ferryl hemes.Biochemistry. 2008 May 6;47(18):5147-55. doi: 10.1021/bi702514d. Epub 2008 Apr 12. Biochemistry. 2008. PMID: 18407661
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
-
Nature of the FeO2 bonding in myoglobin and hemoglobin: A new molecular paradigm.Prog Biophys Mol Biol. 2006 May-Jun;91(1-2):83-162. doi: 10.1016/j.pbiomolbio.2005.04.001. Epub 2005 Jun 9. Prog Biophys Mol Biol. 2006. PMID: 16005052 Review.
Cited by
-
Solving the scalability issue in quantum-based refinement: Q|R#1.Acta Crystallogr D Struct Biol. 2017 Dec 1;73(Pt 12):1020-1028. doi: 10.1107/S2059798317016746. Epub 2017 Nov 30. Acta Crystallogr D Struct Biol. 2017. PMID: 29199981 Free PMC article.
-
Does the crystal structure of vanadium nitrogenase contain a reaction intermediate? Evidence from quantum refinement.J Biol Inorg Chem. 2020 Sep;25(6):847-861. doi: 10.1007/s00775-020-01813-z. Epub 2020 Aug 27. J Biol Inorg Chem. 2020. PMID: 32856107 Free PMC article.
-
Conformational Analysis of Free and Bound Retinoic Acid.J Chem Theory Comput. 2012 Apr 10;8(4):1436-1448. doi: 10.1021/ct200813q. Epub 2012 Feb 24. J Chem Theory Comput. 2012. PMID: 22844234 Free PMC article.
-
Correlating Structure with Spectroscopy in Ascorbate Peroxidase Compound II.J Am Chem Soc. 2024 Apr 10;146(14):9640-9656. doi: 10.1021/jacs.3c13169. Epub 2024 Mar 26. J Am Chem Soc. 2024. PMID: 38530124 Free PMC article.
-
XFEL Crystal Structures of Peroxidase Compound II.Angew Chem Weinheim Bergstr Ger. 2021 Jun 21;133(26):14699-14706. doi: 10.1002/ange.202103010. Epub 2021 May 19. Angew Chem Weinheim Bergstr Ger. 2021. PMID: 38505375 Free PMC article.
References
-
- Ahlrichs, R., M. Bär, H.-P. Baron, R. Bauernschmitt, S. Böcker, M. Ehrig, K. Eichkorn, S. Elliott, F. Haase, M. Häser, H. Horn, C. Huber, C. Kölmel, M. Kollwitz, C. Ochsenfeld, H. Öhm, A. Schäfer, U. Schneider, O. Treutler, M. von Arnim, F. Weigend, P. Weis, and H. Weiss. 2000. TURBOMOLE. Universität Karlsruhe, Germany.
-
- Andersson, L. A., and J. H. Dawson. 1991. EXAFS spectroscopy of heme-containing oxygenases and peroxidases. Struct. Bond. 74:1–40.
-
- Antony, J., M. Grodzicki, and A. X. Trautwein. 1997. Local density functional study of oxoironIV porphyrin complexes and their one-electron oxidized derivatives. Axial ligand effects. J. Phys. Chem. A. 101:2692–2701.
-
- Bashford, D. 1997. An object-oriented programming suite for electrostatic effects in biological molecules. In Scientific Computing in Object-Oriented Parallel Environments, Lecture Notes in Computer Science, Vol. 134. Y. Ishikawa, R.R. Oldehoeft, J.V.W. Reynders, and M. Tholburn, editors. Springer, Berlin, Germany. 233–240.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

