RhoE inhibits cell cycle progression and Ras-induced transformation
- PMID: 15340047
- PMCID: PMC515038
- DOI: 10.1128/MCB.24.18.7829-7840.2004
RhoE inhibits cell cycle progression and Ras-induced transformation
Abstract
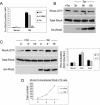
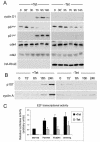
Rho GTPases are major regulators of cytoskeletal dynamics, but they also affect cell proliferation, transformation, and oncogenesis. RhoE, a member of the Rnd subfamily that does not detectably hydrolyze GTP, inhibits RhoA/ROCK signaling to promote actin stress fiber and focal adhesion disassembly. We have generated fibroblasts with inducible RhoE expression to investigate the role of RhoE in cell proliferation. RhoE expression induced a loss of stress fibers and cell rounding, but these effects were only transient. RhoE induction inhibited cell proliferation and serum-induced S-phase entry. Neither ROCK nor RhoA inhibition accounted for this response. Consistent with its inhibitory effect on cell cycle progression, RhoE expression was induced by cisplatin, a DNA damage-inducing agent. RhoE-expressing cells failed to accumulate cyclin D1 or p21(cip1) protein or to activate E2F-regulated genes in response to serum, although ERK, PI3-K/Akt, FAK, Rac, and cyclin D1 transcription was activated normally. The expression of proteins that bypass the retinoblastoma (pRb) family cell cycle checkpoint, including human papillomavirus E7, adenovirus E1A, and cyclin E, rescued cell cycle progression in RhoE-expressing cells. RhoE also inhibited Ras- and Raf-induced fibroblast transformation. These results indicate that RhoE inhibits cell cycle progression upstream of the pRb checkpoint.
Figures



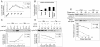



Similar articles
-
Down-regulation of cyclin A gene expression upon genotoxic stress correlates with reduced binding of free E2F to the promoter.Cell Growth Differ. 1997 Jun;8(6):699-710. Cell Growth Differ. 1997. PMID: 9186003
-
Function and regulation of RhoE.Biochem Soc Trans. 2005 Aug;33(Pt 4):649-51. doi: 10.1042/BST0330649. Biochem Soc Trans. 2005. PMID: 16042565 Review.
-
K-ras modulates the cell cycle via both positive and negative regulatory pathways.Oncogene. 1997 May 29;14(21):2595-607. doi: 10.1038/sj.onc.1201105. Oncogene. 1997. PMID: 9191059
-
Regulation of cell cycle entry and G1 progression by CSF-1.Mol Reprod Dev. 1997 Jan;46(1):11-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. Mol Reprod Dev. 1997. PMID: 8981358 Review.
-
RhoE interferes with Rb inactivation and regulates the proliferation and survival of the U87 human glioblastoma cell line.Exp Cell Res. 2007 Feb 15;313(4):719-31. doi: 10.1016/j.yexcr.2006.11.006. Epub 2006 Nov 18. Exp Cell Res. 2007. PMID: 17182035
Cited by
-
Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions.Cancer Res. 2009 Mar 15;69(6):2224-33. doi: 10.1158/0008-5472.CAN-08-3201. Epub 2009 Feb 24. Cancer Res. 2009. PMID: 19244113 Free PMC article.
-
Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling.Neuron. 2011 Mar 24;69(6):1069-84. doi: 10.1016/j.neuron.2011.02.018. Neuron. 2011. PMID: 21435554 Free PMC article.
-
Role of non-genomic androgen signalling in suppressing proliferation of fibroblasts and fibrosarcoma cells.Cell Death Dis. 2014 Dec 4;5(12):e1548. doi: 10.1038/cddis.2014.497. Cell Death Dis. 2014. PMID: 25476896 Free PMC article.
-
Downregulation of RND3/RhoE in glioblastoma patients promotes tumorigenesis through augmentation of notch transcriptional complex activity.Cancer Med. 2015 Sep;4(9):1404-16. doi: 10.1002/cam4.484. Epub 2015 Jun 24. Cancer Med. 2015. PMID: 26108681 Free PMC article.
-
Gem associates with Ezrin and acts via the Rho-GAP protein Gmip to down-regulate the Rho pathway.Mol Biol Cell. 2007 Apr;18(4):1242-52. doi: 10.1091/mbc.e06-06-0510. Epub 2007 Jan 31. Mol Biol Cell. 2007. PMID: 17267693 Free PMC article.
References
-
- Akashi, H., H. J. Han, M. Iizaka, and Y. Nakamura. 2000. Growth-suppressive effect of non-steroidal anti-inflammatory drugs on 11 colon-cancer cell lines and fluorescence differential display of genes whose expression is influenced by sulindac. Int. J. Cancer 88:873-880. - PubMed
-
- Assoian, R. K., and M. A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48-53. - PubMed
-
- Bektic, J., O. A. Wrulich, G. Dobler, K. Kofler, F. Ueberall, Z. Culig, G. Bartsch, and H. Klocker. 2004. Identification of genes involved in estrogenic action in the human prostate using microarray analysis. Genomics 83:34-44. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
