Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes
- PMID: 15340055
- PMCID: PMC515037
- DOI: 10.1128/MCB.24.18.7931-7940.2004
Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes
Abstract
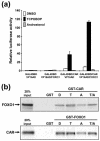
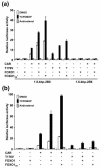
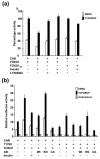
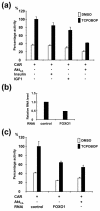
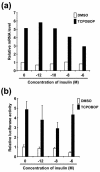
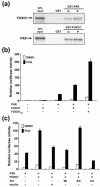
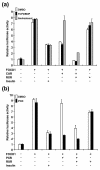
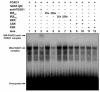
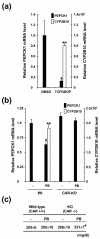
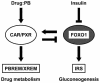
The nuclear receptors CAR and PXR activate hepatic genes in response to therapeutic drugs and xenobiotics, leading to the induction of drug-metabolizing enzymes, such as cytochrome P450. Insulin inhibits the ability of FOXO1 to express genes encoding gluconeogenic enzymes. Induction by drugs is known to be decreased by insulin, whereas gluconeogenic activity is often repressed by treatment with certain drugs, such as phenobarbital (PB). Performing cell-based transfection assays with drug-responsive and insulin-responsive enhancers, glutathione S-transferase pull down, RNA interference (RNAi), and mouse primary hepatocytes, we examined the molecular mechanism by which nuclear receptors and FOXO1 could coordinately regulate both enzyme pathways. FOXO1 was found to be a coactivator to CAR- and PXR-mediated transcription. In contrast, CAR and PXR, acting as corepressors, downregulated FOXO1-mediated transcription in the presence of their activators, such as 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) and pregnenolone 16alpha-carbonitrile, respectively. A constitutively active mutant of the insulin-responsive protein kinase Akt, but not the kinase-negative mutant, effectively blocked FOXO1 activity in cell-based assays. Thus, insulin could repress the receptors by activating the Akt-FOXO1 signal, whereas drugs could interfere with FOXO1-mediated transcription by activating CAR and/or PXR. Treatment with TCPOBOP or PB decreased the levels of phosphoenolpyruvate carboxykinase 1 mRNA in mice but not in Car(-/-) mice. We conclude that FOXO1 and the nuclear receptors reciprocally coregulate their target genes, modulating both drug metabolism and gluconeogenesis.
Figures
Similar articles
-
RNA-Seq reveals common and unique PXR- and CAR-target gene signatures in the mouse liver transcriptome.Biochim Biophys Acta. 2016 Sep;1859(9):1198-1217. doi: 10.1016/j.bbagrm.2016.04.010. Epub 2016 Apr 23. Biochim Biophys Acta. 2016. PMID: 27113289 Free PMC article.
-
Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene.Biochem J. 2007 Nov 1;407(3):373-81. doi: 10.1042/BJ20070481. Biochem J. 2007. PMID: 17635106 Free PMC article.
-
The roles of nuclear receptors CAR and PXR in hepatic energy metabolism.Drug Metab Pharmacokinet. 2008;23(1):8-13. doi: 10.2133/dmpk.23.8. Drug Metab Pharmacokinet. 2008. PMID: 18305370 Review.
-
Xenobiotic-induced hepatocyte proliferation associated with constitutive active/androstane receptor (CAR) or peroxisome proliferator-activated receptor α (PPARα) is enhanced by pregnane X receptor (PXR) activation in mice.PLoS One. 2013 Apr 23;8(4):e61802. doi: 10.1371/journal.pone.0061802. Print 2013. PLoS One. 2013. PMID: 23626729 Free PMC article.
-
P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR.Arch Biochem Biophys. 1999 Sep 1;369(1):11-23. doi: 10.1006/abbi.1999.1351. Arch Biochem Biophys. 1999. PMID: 10462436 Review.
Cited by
-
Strategies for developing pregnane X receptor antagonists: Implications from metabolism to cancer.Med Res Rev. 2020 May;40(3):1061-1083. doi: 10.1002/med.21648. Epub 2019 Nov 28. Med Res Rev. 2020. PMID: 31782213 Free PMC article. Review.
-
A selective gut bacterial bile salt hydrolase alters host metabolism.Elife. 2018 Jul 17;7:e37182. doi: 10.7554/eLife.37182. Elife. 2018. PMID: 30014852 Free PMC article.
-
The Exposome and Toxicology: A Win-Win Collaboration.Toxicol Sci. 2022 Feb 28;186(1):1-11. doi: 10.1093/toxsci/kfab149. Toxicol Sci. 2022. PMID: 34878125 Free PMC article. Review.
-
Activation of the constitutive androstane receptor inhibits gluconeogenesis without affecting lipogenesis or fatty acid synthesis in human hepatocytes.Toxicol Appl Pharmacol. 2014 Aug 15;279(1):33-42. doi: 10.1016/j.taap.2014.05.009. Epub 2014 May 27. Toxicol Appl Pharmacol. 2014. PMID: 24878338 Free PMC article.
-
A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway.J Biol Chem. 2006 May 26;281(21):15013-20. doi: 10.1074/jbc.M511116200. Epub 2006 Mar 23. J Biol Chem. 2006. PMID: 16556603 Free PMC article.
References
-
- Dixon, R. L., L. G. Hart, and J. R. Fouts. 1961. The metabolism of drugs by liver microsomes from alloxan-diabetic rats. J. Pharmacol. Exp. Ther. 133:7-11. - PubMed
-
- Dowell, P., T. C. Otto, S. Adi, and M. D. Lane. 2003. Convergence of proxisome proliferator-activated receptor-γ and Foxo1 signaling pathway. J. Biol. Chem. 278:45485-45491. - PubMed
-
- Goodwin, B., M. R. Redinbo, and S. A. Kliewer. 2002. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu. Rev. Pharmacol. Toxicol. 42:1-23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
