Innate immune responses in peptidoglycan recognition protein L-deficient mice
- PMID: 15340057
- PMCID: PMC515053
- DOI: 10.1128/MCB.24.18.7949-7957.2004
Innate immune responses in peptidoglycan recognition protein L-deficient mice
Abstract
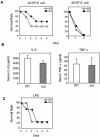
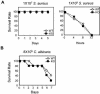
Peptidoglycan recognition proteins (PGRPs) constitute a family of innate immune recognition molecules. In Drosophila, distinct PGRPs bind to peptidoglycans on gram-positive or gram-negative bacteria and provide essential signals upstream of the Toll and Imd pathways required for immunity against infection. Four PGRPs, PGRP-L, -S, -Ialpha, and -Ibeta, are expressed from three genes in mammals. In this paper, we provide direct evidence that the longest family member, PGRP-L, is a secreted serum protein with the capacity to multimerize. Using gene targeting to create PGRP-L-deficient mice, we demonstrate little contribution by PGRP-L to systemic challenge using gram-negative bacteria (Escherichia coli, slightly less susceptible), Gram-positive bacteria (Staphylococcus aureus), or yeast (Candida albicans). Peritoneal macrophages from PGRP-L-deficient mice produced decreased amounts of the inflammatory cytokines interleukin 6 and tumor necrosis factor alpha when stimulated with E. coli or lipopolysaccharide, but comparable amounts when stimulated with S. aureus, C. albicans, or their cell wall components. Additionally, these cells produced similar amounts of cytokines when challenged with gram-positive or -negative peptidoglycans. In contrast to its critical role in immunity in flies, PGRP-L is largely dispensable for mammalian immunity against bacteria and fungi.
Figures



Similar articles
-
Peptidoglycan recognition proteins: on and off switches for innate immunity.Immunol Rev. 2004 Apr;198:83-96. doi: 10.1111/j.0105-2896.2004.0120.x. Immunol Rev. 2004. PMID: 15199956 Review.
-
Tissue- and ligand-specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE.J Immunol. 2012 Aug 15;189(4):1886-97. doi: 10.4049/jimmunol.1201022. Epub 2012 Jul 6. J Immunol. 2012. PMID: 22772451
-
Drosophila immunity: analysis of PGRP-SB1 expression, enzymatic activity and function.PLoS One. 2011 Feb 18;6(2):e17231. doi: 10.1371/journal.pone.0017231. PLoS One. 2011. PMID: 21364998 Free PMC article.
-
Peptidoglycan recognition protein-peptidoglycan complexes increase monocyte/macrophage activation and enhance the inflammatory response.Immunology. 2015 Jul;145(3):429-42. doi: 10.1111/imm.12460. Epub 2015 Apr 16. Immunology. 2015. PMID: 25752767 Free PMC article.
-
[Intra- and extracellular recognition of pathogens and activation of innate immunity].Yakugaku Zasshi. 2006 Dec;126(12):1213-8. doi: 10.1248/yakushi.126.1213. Yakugaku Zasshi. 2006. PMID: 17139146 Review. Japanese.
Cited by
-
Peptidoglycan Recognition Protein 2 Regulates Neutrophil Recruitment Into the Lungs After Streptococcus pneumoniae Infection.Front Microbiol. 2019 Feb 19;10:199. doi: 10.3389/fmicb.2019.00199. eCollection 2019. Front Microbiol. 2019. PMID: 30837960 Free PMC article.
-
Phagocytes containing a disease-promoting Toll-like receptor/Nod ligand are present in the brain during demyelinating disease in primates.Am J Pathol. 2006 Nov;169(5):1671-85. doi: 10.2353/ajpath.2006.060143. Am J Pathol. 2006. PMID: 17071591 Free PMC article.
-
The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior.Mol Psychiatry. 2017 Feb;22(2):257-266. doi: 10.1038/mp.2016.182. Epub 2016 Nov 15. Mol Psychiatry. 2017. PMID: 27843150 Free PMC article.
-
LysMD3 is a type II membrane protein without an in vivo role in the response to a range of pathogens.J Biol Chem. 2018 Apr 20;293(16):6022-6038. doi: 10.1074/jbc.RA117.001246. Epub 2018 Mar 1. J Biol Chem. 2018. PMID: 29496999 Free PMC article.
-
Staphylococcus aureus Promotes Smed-PGRP-2/Smed-setd8-1 Methyltransferase Signalling in Planarian Neoblasts to Sensitize Anti-bacterial Gene Responses During Re-infection.EBioMedicine. 2017 Jun;20:150-160. doi: 10.1016/j.ebiom.2017.04.031. Epub 2017 Apr 24. EBioMedicine. 2017. PMID: 28456423 Free PMC article.
References
-
- Choe, K. M., T. Werner, S. Stoven, D. Hultmark, and K. V. Anderson. 2002. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296:359-362. - PubMed
-
- De Pauw, P., C. Neyt, E. Vanderwinkel, R. Wattiez, and P. Falmagne. 1995. Characterization of human serum N-acetylmuramyl-L-alanine amidase purified by affinity chromatography. Protein Exp. Purif. 6:371-378. - PubMed
-
- Dziarski, R., K. A. Platt, E. Gelius, H. Steiner, and D. Gupta. 2003. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 102:689-697. - PubMed
-
- Gelius, E., C. Persson, J. Karlsson, and H. Steiner. 2003. A mammalian peptidoglycan recognition protein with N-acetylmuramoyl-L-alanine amidase activity. Biochem. Biophys. Res. Commun. 306:988-994. - PubMed
-
- Georgel, P., S. Naitza, C. Kappler, D. Ferrandon, D. Zachary, C. Swimmer, C. Kopczynski, G. Duyk, J. M. Reichhart, and J. A. Hoffmann. 2001. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell 1:503-514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
