Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility
- PMID: 15340140
- PMCID: PMC518783
- DOI: 10.1073/pnas.0405580101
Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility
Abstract
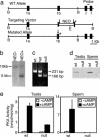
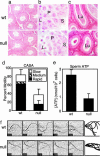
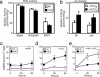
An unusual cAMP signaling system mediates many of the events that prepare spermatozoa to meet the egg. Its components include the atypical, bicarbonate-stimulated, sperm adenylyl cyclase and a cAMP-dependent protein kinase (PKA) with the unique catalytic subunit termed Calpha(2) or C(s). We generated mice that lack Calpha(2) to determine its importance in the events downstream of cAMP production. Male Calpha(2) null mice produce normal numbers of sperm that swim spontaneously in vitro. Thus, Calpha(2) has no required role in formation of a functional flagellum or the initiation of motility. In contrast, we find that Calpha(2) is required for bicarbonate to speed the flagellar beat and facilitate Ca(2+) entry channels. In addition, Calpha(2) is needed for the protein tyrosine phosphorylation that occurs late in the sequence of sperm maturation and for a negative feedback control of cAMP production, revealed here. Consistent with these specific defects in several important sperm functions, Calpha(2) null males are infertile despite normal mating behavior. These results define several crucial roles of PKA in sperm cell biology, bringing together both known and unique PKA-mediated events that are necessary for male fertility.
Figures


References
-
- Boatman, D. E. & Robbins, R. S. (1991) Biol. Reprod. 44, 806–813. - PubMed
-
- Okamura, N., Tajima, Y., Soejima, A., Masuda, H. & Sugita, Y. (1985) J. Biol. Chem. 260, 9699–9705. - PubMed
-
- Gadella, B. M. & Harrison, R. A. (2000) Development (Cambridge, U.K.) 127, 2407–2420. - PubMed
-
- Wennemuth, G., Carlson, A. E., Harper, A. J. & Babcock, D. F. (2003) Development (Cambridge, U.K.) 130, 1317–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

