Universality in intermediary metabolism
- PMID: 15340153
- PMCID: PMC516543
- DOI: 10.1073/pnas.0404922101
Universality in intermediary metabolism
Abstract
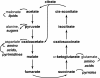
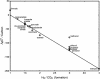
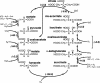
We analyze the stoichiometry, energetics, and reaction concentration dependence of the reductive tricarboxylic acid (rTCA) cycle as a universal and possibly primordial metabolic core. The rTCA reaction sequence is a network-autocatalytic cycle along the relaxation pathway for redox couples in nonequilibrium reducing environments, which provides starting organic compounds for the synthesis of all major classes of biomolecules. The concentration dependence of its reactions suggests it as a precellular bulk process. We propose that rTCA is statistically favored among competing redox relaxation pathways under early-earth conditions and that this feature drove its emergence and also accounts for its evolutionary robustness and universality. The ability to enhance the rate of core reactions creates an energetic basis for selection of subsequent layers of biological complexity.
Figures
References
-
- Wilson, K. G. & Kogut, J. (1974) Phys. Rep., Phys. Lett. 12C, 75–200.
-
- Miller, S. L. & Orgel, L. E. (1974) The Origins of Life on the Earth (Prentice-Hall, Engelwood Cliffs, NJ).
-
- Fry, I. (2000) The Emergence of Life on Earth: A Historical and Scientific Overview, (Rutgers Univ. Press, New Brunswick, NJ).
-
- Whittet, D. C. B. (1997) Origins Life Evol. Biosphere 27, 249–262. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

