Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development
- PMID: 15340492
- PMCID: PMC514883
- DOI: 10.1371/journal.pbio.0020257
Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development
Abstract
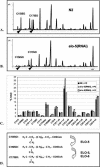
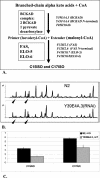
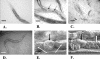
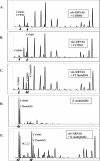
Monomethyl branched-chain fatty acids (mmBCFAs) are commonly found in many organisms from bacteria to mammals. In humans, they have been detected in skin, brain, blood, and cancer cells. Despite a broad distribution, mmBCFAs remain exotic in eukaryotes, where their origin and physiological roles are not understood. Here we report our study of the function and regulation of mmBCFAs in Caenorhabditis elegans, combining genetics, gas chromatography, and DNA microarray analysis. We show that C. elegans synthesizes mmBCFAs de novo and utilizes the long-chain fatty acid elongation enzymes ELO-5 and ELO-6 to produce two mmBCFAs, C15ISO and C17ISO. These mmBCFAs are essential for C. elegans growth and development, as suppression of their biosynthesis results in a growth arrest at the first larval stage. The arrest is reversible and can be overcome by feeding the arrested animals with mmBCFA supplements. We show not only that the levels of C15ISO and C17ISO affect the expression of several genes, but also that the activities of some of these genes affect biosynthesis of mmBCFAs, suggesting a potential feedback regulation. One of the genes, lpd-1, encodes a homolog of a mammalian sterol regulatory element-binding protein (SREBP 1c). We present results suggesting that elo-5 and elo-6 may be transcriptional targets of LPD-1. This study exposes unexpected and crucial physiological functions of C15ISO and C17ISO in C. elegans and suggests a potentially important role for mmBCFAs in other eukaryotes.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



Similar articles
-
Peroxisome protein transportation affects metabolism of branched-chain fatty acids that critically impact growth and development of C. elegans.PLoS One. 2013 Sep 27;8(9):e76270. doi: 10.1371/journal.pone.0076270. eCollection 2013. PLoS One. 2013. PMID: 24086720 Free PMC article.
-
P-type ATPase TAT-2 negatively regulates monomethyl branched-chain fatty acid mediated function in post-embryonic growth and development in C. elegans.PLoS Genet. 2009 Aug;5(8):e1000589. doi: 10.1371/journal.pgen.1000589. Epub 2009 Aug 7. PLoS Genet. 2009. PMID: 19662161 Free PMC article.
-
A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans.Genes Dev. 2008 Aug 1;22(15):2102-10. doi: 10.1101/gad.1692008. Genes Dev. 2008. PMID: 18676815 Free PMC article.
-
Monomethyl branched-chain fatty acids-a pearl dropped in the ocean.Crit Rev Food Sci Nutr. 2024;64(25):9045-9057. doi: 10.1080/10408398.2023.2207655. Epub 2023 May 4. Crit Rev Food Sci Nutr. 2024. PMID: 37140184 Review.
-
Dietary sources of branched-chain fatty acids and their biosynthesis, distribution, and nutritional properties.Food Chem. 2024 Jan 15;431:137158. doi: 10.1016/j.foodchem.2023.137158. Epub 2023 Aug 14. Food Chem. 2024. PMID: 37604010 Review.
Cited by
-
Fatty acids composition of Caenorhabditis elegans using accurate mass GCMS-QTOF.J Environ Sci Health B. 2016 Aug 2;51(8):546-52. doi: 10.1080/03601234.2016.1170555. Epub 2016 May 11. J Environ Sci Health B. 2016. PMID: 27166662 Free PMC article.
-
Regulation of maternal phospholipid composition and IP(3)-dependent embryonic membrane dynamics by a specific fatty acid metabolic event in C. elegans.Genes Dev. 2012 Mar 15;26(6):554-66. doi: 10.1101/gad.187054.112. Genes Dev. 2012. PMID: 22426533 Free PMC article.
-
Evolutionarily related host and microbial pathways regulate fat desaturation in C. elegans.Nat Commun. 2024 Feb 19;15(1):1520. doi: 10.1038/s41467-024-45782-2. Nat Commun. 2024. PMID: 38374083 Free PMC article.
-
The influence of bacterial diet on fat storage in C. elegans.PLoS One. 2009 Oct 21;4(10):e7545. doi: 10.1371/journal.pone.0007545. PLoS One. 2009. PMID: 19844570 Free PMC article.
-
Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production.Gut. 2016 Jan;65(1):63-72. doi: 10.1136/gutjnl-2014-308209. Epub 2014 Nov 26. Gut. 2016. PMID: 25431456 Free PMC article.
References
-
- Ailion M, Toker A, Thomas JH, Ruvkun G, Tissenbaum HA. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans . Genes Dev. 1999;13:1438–1452. - PubMed
-
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. - PubMed
-
- Aungst BJ. Structure/effect studies of fatty acid isomers as skin penetration enhancers and skin irritants. Pharm Res. 1989;6:244–247. - PubMed
-
- Batrakov SG, Mosezhnyi AE, Ruzhitsky AO, Sheichenko VI, Nikitin DI. The polar-lipid composition of the sphingolipid-producing bacterium Flectobacillus major . Biochim Biophys Acta. 2000;1484:225–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

