Cellular antitumor immune response to a branched lysine multiple antigenic peptide containing epitopes of a common tumor-specific antigen in a rat glioma model
- PMID: 15340764
- PMCID: PMC11032903
- DOI: 10.1007/s00262-004-0576-y
Cellular antitumor immune response to a branched lysine multiple antigenic peptide containing epitopes of a common tumor-specific antigen in a rat glioma model
Abstract
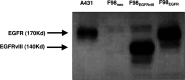
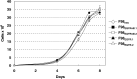
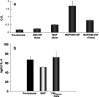
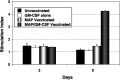
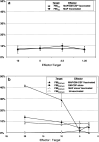
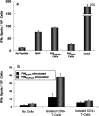
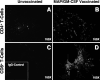
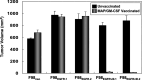
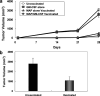
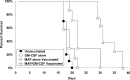
Human malignant gliomas contain epidermal growth factor receptor (EGFR) gene mutations that encode tumor-associated antigens (TAAs) that can be targeted using immunological techniques. One EGFR mutant gene (EGFRvIII) encodes a protein with an epitope that is not found in normal tissues. A number of studies have focused on this unique epitope as a potential target for tumor vaccines. In the present study, we examined the cellular immune effects of a peptide containing multiple copies of the unique EGFRvIII epitope linked together by way of a lysine bridge. Fischer rats were vaccinated with an EGFRvIII multiple antigenic peptide (MAP). While vaccination produced a humoral immune response, anti-MAP antibody production was not accompanied by expression of the Th2 response cytokine IL-4. In MAP/GM-CSF vaccinated animals, a cellular immune response was detected in association with the appearance of CD4+ and CD8+ T cells at the tumor site. Splenocytes and CD8+ T cells from vaccinated rats produced the Th1 cytokine IFN-gamma in vitro in response to stimulation by rat glioma cells expressing EGFRvIII, but not by those expressing wild-type EGFR. MAP vaccine also induced a specific lytic antitumor CTL immune response against F98 glioma cells expressing EGFRvIII, but not against F98 cells expressing either wild-type EGFR or no receptor. The in vivo growth of F98(EGFRvIII) cells was attenuated in vaccinated rats; whereas, growth of F98(EGFR) cells was not. The median survival of vaccinated rats was increased 72% over that of unvaccinated controls challenged with intracerebral F98(EGFRvIII) tumor implants. Therefore, MAP vaccination produced a predominantly cellular antitumor immune response directed against F98 gliomas expressing the EGFRvIII target antigen. The potent immunosuppressive effects of F98 glioma cells mimic the human disease and make this particular tumor model useful for studying immunotherapeutic approaches to malignant gliomas.
Figures


References
-
- Wingo PA, Tong T, Bolden S. Cancer statistics. CA Cancer J Clin. 1995;45:8–30. - PubMed
-
- McKinley B, Michalek A, Plunkett RJ, Fenstermaker RA. The impact of age and sex on the incidence of glial tumors in New York State from 1976 to 1995. J Neurosurg. 2000;93:932–939. - PubMed
-
- Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

