Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]
- PMID: 15341662
- PMCID: PMC517724
- DOI: 10.1186/1472-6882-4-12
Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]
Abstract
Background: The morbidity and mortality associated with depression are considerable and continue to increase. Depression currently ranks fourth among the major causes of disability worldwide, after lower respiratory infections, prenatal conditions, and HIV/AIDS. Crocus sativus L. is used to treat depression. Many medicinal plants textbooks refer to this indication whereas there is no evidence-based document. Our objective was to compare the efficacy of stigmas of Crocus sativus (saffron) with imipramine in the treatment of mild to moderate depression in a 6-week pilot double-blind randomized trial.
Methods: Thirty adult outpatients who met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition for major depression based on the structured clinical interview for DSM IV participated in the trial. Patients have a baseline Hamilton Rating Scale for Depression score of at least 18. In this double-blind, single-center trial, patients were randomly assigned to receive capsule of saffron 30 mg/day (TDS) (Group 1) and capsule of imipramine 100 mg/day (TDS) (Group 2) for a 6-week study.
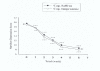
Results: Saffron at this dose was found to be effective similar to imipramine in the treatment of mild to moderate depression (F = 2.91, d.f. = 1, P = 0.09). In the imipramine group anticholinergic effects such as dry mouth and also sedation were observed more often that was predictable.
Conclusion: The main overall finding from this study is that saffron may be of therapeutic benefit in the treatment of mild to moderate depression. To the best of our knowledge this is the first clinical trial that supports this indication for saffron. A large-scale trial with placebo control is warranted.
Figures
References
-
- Judd L. Mood disorders in the general population represent an important and worldwide public health problem. Int J Clin Psychopharm. 1995;10:5–10. - PubMed
-
- Richelson E. Pharmacology of antidepressants-characteristic of the ideal drug. Mayo Clin Proc. 1994;69:1069–1081. - PubMed
-
- Donoghue JM, Tylee A. The treatment of depression: prescribing patterns of antidepressants in primary care in the UK. Br J Psychiatry. 1996;168:164–168. - PubMed
-
- MacDonald TM. Treatment of depression: prescription for success? Prim Care Psychiatry. 1997;3:7–10.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

