Attenuation of estrogen receptor alpha-mediated transcription through estrogen-stimulated recruitment of a negative elongation factor
- PMID: 15342491
- PMCID: PMC515291
- DOI: 10.1101/gad.1214104
Attenuation of estrogen receptor alpha-mediated transcription through estrogen-stimulated recruitment of a negative elongation factor
Abstract
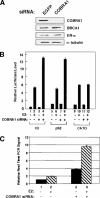
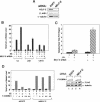
Estrogen receptor alpha (ERalpha) signaling is paramount for normal mammary gland development and function and the repression of breast cancer. ERalpha function in gene regulation is mediated by a number of coactivators and corepressors, most of which are known to modify chromatin structure and/or influence the assembly of the regulatory complexes at the level of transcription initiation. Here we describe a novel mechanism of attenuating the ERalpha activity. We show that cofactor of BRCA1 (COBRA1), an integral subunit of the human negative elongation factor (NELF), directly binds to ERalpha and represses ERalpha-mediated transcription. Reduction of the endogenous NELF proteins in breast cancer cells using small interfering RNA results in elevated ERalpha-mediated transcription and enhanced cell proliferation. Chromatin immunoprecipitation reveals that recruitment of COBRA1 and the other NELF subunits to endogenous ERalpha-responsive promoters is greatly stimulated upon estrogen treatment. Interestingly, COBRA1 does not affect the estrogen-dependent assembly of transcription regulatory complexes at the ERalpha-regulated promoters. Rather, it causes RNA polymerase II (RNAPII) to pause at the promoter-proximal region, which is consistent with its in vitro biochemical activity. Therefore, our in vivo work defines the first corepressor of nuclear receptors that modulates ERalpha-dependent gene expression by stalling RNAPII. We suggest that this new level of regulation may be important to control the duration and magnitude of a rapid and reversible hormonal response.
Figures





References
-
- Ali S. and Coombes, R.C. 2002. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2: 101-112. - PubMed
-
- Augereau P., Miralles, F., Cavailles, V., Gaudelet, C., Parker, M., and Rochefort, H. 1994. Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Mol. Endocrinol. 8: 693-703. - PubMed
-
- Baniahmad C., Nawaz, Z., Baniahmad, A., Gleeson, M.A., Tsai, M.J., and O'Malley, B.W. 1995. Enhancement of human estrogen receptor activity by SPT6: A potential coactivator. Mol. Endocrinol. 9: 34-43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
