Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver
- PMID: 15342726
- PMCID: PMC6729629
- DOI: 10.1523/JNEUROSCI.5328-03.2004
Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver
Abstract
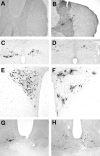
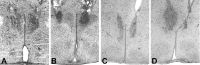
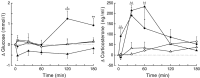
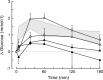
Daily peak plasma glucose concentrations are attained shortly before awakening. Previous experiments indicated an important role for the biological clock, located in the suprachiasmatic nuclei (SCN), in the genesis of this anticipatory rise in plasma glucose concentrations by controlling hepatic glucose production. Here, we show that stimulation of NMDA receptors, or blockade of GABA receptors in the paraventricular nucleus of the hypothalamus (PVN) of conscious rats, caused a pronounced increase in plasma glucose concentrations. The local administration of TTX in brain areas afferent to the PVN revealed that an important part of the inhibitory inputs to the PVN was derived from the SCN. Using a transneuronal viral-tracing technique, we showed that the SCN is connected to the liver via both branches of the autonomic nervous system (ANS). The combination of a blockade of GABA receptors in the PVN with selective removal of either the sympathetic or parasympathetic branch of the hepatic ANS innervation showed that hyperglycemia produced by PVN stimulation was primarily attributable to an activation of the sympathetic input to the liver. We propose that the daily rise in plasma glucose concentrations is caused by an SCN-mediated withdrawal of GABAergic inputs to sympathetic preautonomic neurons in the PVN, resulting in an increased hepatic glucose production. The remarkable resemblance of the presently proposed control mechanism to that described previously for the control of daily melatonin rhythm suggests that the GABAergic control of sympathetic preautonomic neurons in the PVN is an important pathway for the SCN to control peripheral physiology.
Figures




References
-
- Abe M, Saito M, Shimazu T (1989) Neuropeptide Y and norepinephrine injected into the paraventricular nucleus of the hypothalamus activate endocrine pancreas. Biomed Res 10: 431-436.
-
- Boden G, Chen X, Urbain JL (1996) Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45: 1044-1050. - PubMed
-
- Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE (1984) Demonstration of a dawn phenomenon in normal human volunteers. Diabetes 33: 1150-1153. - PubMed
-
- Buijs RM, Markman M, Nunes-Cardoso B, Hou Y-X, Shinn S (1993) Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol 335: 42-54. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
