Olfactory receptor proteins in axonal processes of chemosensory neurons
- PMID: 15342743
- PMCID: PMC6729612
- DOI: 10.1523/JNEUROSCI.2588-04.2004
Olfactory receptor proteins in axonal processes of chemosensory neurons
Abstract
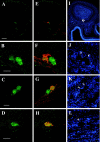
Olfactory receptors are supposed to act not only as molecular sensors for odorants but also as cell recognition molecules guiding the axons of olfactory neurons to their appropriate glomerulus in the olfactory bulb. This concept implies that olfactory receptor proteins are located in sensory cilia and in the axons. To approach this critical issue, antibodies were generated against two peptides, one derived from olfactory receptor mOR256-17, one derived from the "mOR37" subfamily. By means of immunohistochemistry and double-labeling studies using transgenic mouse lines as well as Western blot analyses, it was demonstrated that the newly generated antibodies specifically recognized the receptor proteins. To scrutinize the hypothesis that olfactory receptor proteins may also be present in the axonal processes and the nerve terminals, serial sections through the olfactory bulb were probed with the antibodies. Two glomeruli in each bulb were stained by anti-mOR256-17, one positioned in the medial, one in the lateral hemisphere. Fiber bundles approaching the glomeruli through the outer nerve layer also displayed intense immunofluorescence. A similar picture emerged for the antibody anti-mOR37, a small number of glomeruli in the ventral domain of the bulb was stained. On serial sections through the olfactory bulb of mOR37-transgenic mouse lines, double-labeling experiments demonstrated that distinct immunoreactive glomeruli corresponded to glomeruli that were targeted by neurons expressing a particular member of the mOR37 receptor subfamily. These data indicate that olfactory receptor (OR) proteins are indeed present in the axonal processes and nerve terminals of olfactory sensory neurons, thus supporting the notion that ORs may participate in the molecular processes underlying the fasciculation and targeting of olfactory axons.
Figures






References
-
- Barnea G, O'Donnell S, Mancia F, Sun X, Nemes A, Mendelson M, Axel R (2004) Odorant receptors on axon termini in the brain. Science 304: 1468. - PubMed
-
- Bozza TC, Mombaerts P (2001) Olfactory coding: revealing intrinsic representations of odors. Curr Biol 11: R687-R690. - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microquantites of protein utilizing the principle of protein-dye binding. Anal Biochem 65: 248-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
