Regulation of cell motility by tyrosine phosphorylated villin
- PMID: 15342783
- PMCID: PMC524729
- DOI: 10.1091/mbc.e04-05-0431
Regulation of cell motility by tyrosine phosphorylated villin
Abstract
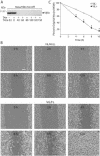
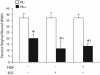
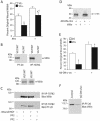
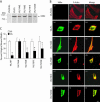
Temporal and spatial regulation of the actin cytoskeleton is vital for cell migration. Here, we show that an epithelial cell actin-binding protein, villin, plays a crucial role in this process. Overexpression of villin in doxycyline-regulated HeLa cells enhanced cell migration. Villin-induced cell migration was modestly augmented by growth factors. In contrast, tyrosine phosphorylation of villin and villin-induced cell migration was significantly inhibited by the src kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) as well as by overexpression of a dominant negative mutant of c-src. These data suggest that phosphorylation of villin by c-src is involved in the actin cytoskeleton remodeling necessary for cell migration. We have previously shown that villin is tyrosine phosphorylated at four major sites. To further investigate the role of tyrosine phosphorylated villin in cell migration, we used phosphorylation site mutants (tyrosine to phenylalanine or tyrosine to glutamic acid) in HeLa cells. We determined that tyrosine phosphorylation at residues 60, 81, and 256 of human villin played an essential role in cell migration as well as in the reorganization of the actin cytoskeleton. Collectively, these studies define how biophysical events such as cell migration are actuated by biochemical signaling pathways involving tyrosine phosphorylation of actin binding proteins, in this case villin.
Figures

Similar articles
-
Regulation of actin dynamics by tyrosine phosphorylation: identification of tyrosine phosphorylation sites within the actin-severing domain of villin.Biochemistry. 2002 Oct 1;41(39):11750-60. doi: 10.1021/bi0263762. Biochemistry. 2002. PMID: 12269817
-
Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton.J Biol Chem. 2001 Sep 28;276(39):36163-7. doi: 10.1074/jbc.C100418200. Epub 2001 Aug 10. J Biol Chem. 2001. PMID: 11500485
-
Obligatory role for phospholipase C-gamma(1) in villin-induced epithelial cell migration.Am J Physiol Cell Physiol. 2007 May;292(5):C1775-86. doi: 10.1152/ajpcell.00420.2006. Epub 2007 Jan 17. Am J Physiol Cell Physiol. 2007. PMID: 17229814
-
The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells?Am J Physiol Gastrointest Liver Physiol. 2002 Sep;283(3):G496-502. doi: 10.1152/ajpgi.00207.2002. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 12181160 Review.
-
Regulation of cell structure and function by actin-binding proteins: villin's perspective.FEBS Lett. 2008 Jun 18;582(14):2128-39. doi: 10.1016/j.febslet.2008.02.040. Epub 2008 Feb 26. FEBS Lett. 2008. PMID: 18307996 Free PMC article. Review.
Cited by
-
Differential effects of lysophosphatidic acid and phosphatidylinositol 4,5-bisphosphate on actin dynamics by direct association with the actin-binding protein villin.J Biol Chem. 2009 Dec 18;284(51):35278-82. doi: 10.1074/jbc.C109.060830. J Biol Chem. 2009. PMID: 19808673 Free PMC article.
-
FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion.Mol Biol Cell. 2011 Apr;22(7):964-75. doi: 10.1091/mbc.E10-08-0725. Epub 2011 Feb 2. Mol Biol Cell. 2011. PMID: 21289086 Free PMC article.
-
Tyrosine 311 is phosphorylated by c-Abl and promotes the apoptotic effect of PKCdelta in glioma cells.Biochem Biophys Res Commun. 2007 Jan 12;352(2):431-6. doi: 10.1016/j.bbrc.2006.11.028. Epub 2006 Nov 16. Biochem Biophys Res Commun. 2007. PMID: 17126298 Free PMC article.
-
Tumor lymphangiogenesis and metastasis to lymph nodes induced by cancer cell expression of podoplanin.Am J Pathol. 2010 Aug;177(2):1004-16. doi: 10.2353/ajpath.2010.090703. Epub 2010 Jul 8. Am J Pathol. 2010. PMID: 20616339 Free PMC article.
-
Loss of villin immunoexpression in colorectal carcinoma is associated with poor differentiation and survival.ISRN Gastroenterol. 2013 Sep 5;2013:679724. doi: 10.1155/2013/679724. eCollection 2013. ISRN Gastroenterol. 2013. PMID: 24083028 Free PMC article.
References
-
- Anderson, R.D., Haskell, R.E., Xia, H., Roessler, B.J., and Davidson, B.L. (2000). A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7, 1034-1038. - PubMed
-
- Arora, P.D., and McCulloch, C.A. (1996). Dependence of fibroblast migration on actin severing activity of gelsolin. J. Biol. Chem. 271, 20516-20523. - PubMed
-
- Arpin, M., Pringault, E., Finidori, J., Garcia, A., Jeltsch, J.M., Vandekerckhove, J., and Louvard, D. (1988). Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J. Cell Biol. 107, 1759-1766. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

