NALT- versus Peyer's-patch-mediated mucosal immunity
- PMID: 15343369
- PMCID: PMC7097243
- DOI: 10.1038/nri1439
NALT- versus Peyer's-patch-mediated mucosal immunity
Abstract
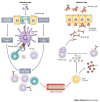
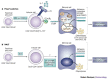
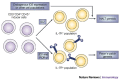
Recent studies indicate that the mechanism of nasopharynx-associated lymphoid tissue (NALT) organogenesis is different from that of other lymphoid tissues. NALT has an important role in the induction of mucosal immune responses, including the generation of T helper 1 and T helper 2 cells, and IgA-committed B cells. Moreover, intranasal immunization can lead to the induction of antigen-specific protective immunity in both the mucosal and systemic immune compartments. Therefore, a greater understanding of the differences between NALT and other organized lymphoid tissues, such as Peyer's patches, should facilitate the development of nasal vaccines.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Mestecky J, Blumberg R, Kiyono H, McGhee JR. Fundamental Immunology. 2003. pp. 965–1020.
-
- Csencsits KL, Jutila MA, Pascual DW. Nasal-associated lymphoid tissue: phenotypic and functional evidence for the primary role of peripheral node addressin in naive lymphocyte adhesion to high endothelial venules in a mucosal site. J. Immunol. 1999;163:1382–1389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

