EVI1 induces myelodysplastic syndrome in mice
- PMID: 15343390
- PMCID: PMC514587
- DOI: 10.1172/JCI21716
EVI1 induces myelodysplastic syndrome in mice
Erratum in
- J Clin Invest. 2005 Aug;115(8):2296
Abstract
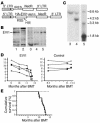
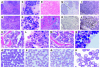
Myelodysplasia is a hematological disease in which genomic abnormalities accumulate in a hematopoietic stem cell leading to severe pancytopenia, multilineage differentiation impairment, and bone marrow (BM) apoptosis. Mortality in the disease results from pancytopenia or transformation to acute myeloid leukemia. There are frequent cytogenetic abnormalities, including deletions of chromosomes 5, 7, or both. Recurring chromosomal translocations in myelodysplasia are rare, but the most frequent are the t(3;3)(q21;q26) and the inv(3)(q21q26), which lead to the inappropriate activation of the EVI1 gene located at 3q26. To better understand the role of EVI1 in this disease, we have generated a murine model of EVI1-positive myelodysplasia by BM infection and transplantation. We find that EVI1 induces a fatal disease of several stages that is characterized by severe pancytopenia. The disease does not progress to acute myeloid leukemia. Comparison of in vitro and in vivo results suggests that EVI1 acts at two levels. The immediate effects of EVI1 are hyperproliferation of BM cells and downregulation of EpoR and c-Mpl, which are important for terminal erythroid differentiation and platelet formation. These defects are not fatal, and the mice survive for about 10 months with compensated hematopoiesis. Over this time, compensation fails, and the mice succumb to fatal peripheral cytopenia.
Figures

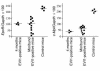
Similar articles
-
The leukemia-associated gene MDS1/EVI1 is a new type of GATA-binding transactivator.Leukemia. 1997 Mar;11(3):352-8. doi: 10.1038/sj.leu.2400584. Leukemia. 1997. PMID: 9067573
-
Methylation and silencing of miRNA-124 by EVI1 and self-renewal exhaustion of hematopoietic stem cells in murine myelodysplastic syndrome.Proc Natl Acad Sci U S A. 2010 May 25;107(21):9783-8. doi: 10.1073/pnas.1004297107. Epub 2010 May 6. Proc Natl Acad Sci U S A. 2010. PMID: 20448201 Free PMC article.
-
[EVI1 and its role in myelodysplastic syndrome, myeloid leukemia and other malignant diseases].Cas Lek Cesk. 2006;145(8):619-24. Cas Lek Cesk. 2006. PMID: 16995417 Review. Czech.
-
Expression of EVI1 in myelodysplastic syndromes and other hematologic malignancies without 3q26 translocations.Blood. 1994 Aug 15;84(4):1243-8. Blood. 1994. PMID: 8049440
-
3q26/EVI1 rearrangements in myeloid hemopathies: a cytogenetic review.Future Oncol. 2015;11(11):1675-86. doi: 10.2217/fon.15.64. Future Oncol. 2015. PMID: 26043219 Review.
Cited by
-
Pathogenetic significance of ecotropic viral integration site-1 in hematological malignancies.Cancer Sci. 2009 Jun;100(6):990-5. doi: 10.1111/j.1349-7006.2009.01152.x. Epub 2009 Mar 9. Cancer Sci. 2009. PMID: 19385966 Free PMC article. Review.
-
-7/7q- syndrome in myeloid-lineage hematopoietic malignancies: attempts to understand this complex disease entity.Oncogene. 2015 May 7;34(19):2413-25. doi: 10.1038/onc.2014.196. Epub 2014 Jul 7. Oncogene. 2015. PMID: 24998854 Review.
-
Evi1 governs Kdm6b-mediated histone demethylation to regulate the Laptm4b-driven mTOR pathway in hematopoietic progenitor cells.J Clin Invest. 2024 Dec 16;134(24):e173403. doi: 10.1172/JCI173403. J Clin Invest. 2024. PMID: 39680456 Free PMC article.
-
Point mutations in two EVI1 Zn fingers abolish EVI1-GATA1 interaction and allow erythroid differentiation of murine bone marrow cells.Mol Cell Biol. 2006 Oct;26(20):7658-66. doi: 10.1128/MCB.00363-06. Epub 2006 Sep 5. Mol Cell Biol. 2006. PMID: 16954386 Free PMC article.
-
Myelodysplastic syndrome and histone deacetylase inhibitors: "to be or not to be acetylated"?J Biomed Biotechnol. 2011;2011:214143. doi: 10.1155/2011/214143. Epub 2011 May 15. J Biomed Biotechnol. 2011. PMID: 21629744 Free PMC article. Review.
References
-
- Mucenski ML, Taylor BA, Copeland NG, Jenkins NA. Chromosomal location of EVI1 in the DNA of AKXD murine myeloid tumors. Oncol. Res. 1988;2:219–233. - PubMed
-
- Morishita K, Parker DS, Mucenski ML, Copeland NG, Ihle JN. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell. 1988;54:831–840. - PubMed
-
- Chakraborty S, Senyuk V, Sitailo S, Chi Y, Nucifora G. Interaction of EVI1 with cAMP-responsive element-binding protein-binding protein (CBP) and p300/CBP-associated factor (P/CAF) results in reversible acetylation of EVI1 and in co-localization in nuclear speckles. J. Biol. Chem. 2001;276:44936–44943. - PubMed
-
- Buonamici S, Chakraborty S, Vitalyi S, Nucifora G. The role of EVI1 in normal and leukemic cells. Blood Cells Mol. Dis. 2003;31:206–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

