Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide
- PMID: 15344905
- PMCID: PMC1134681
- DOI: 10.1042/BJ20041270
Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide
Abstract
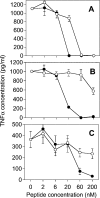
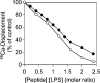
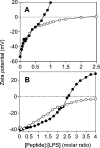
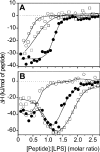
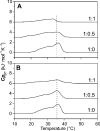
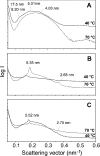
Antibacterial peptide acylation, which mimics the structure of the natural lipopeptide polymyxin B, increases antimicrobial and endotoxin-neutralizing activities. The interaction of the lactoferricin-derived peptide LF11 and its N-terminally acylated analogue, lauryl-LF11, with different chemotypes of bacterial lipopolysaccharide (LPS Re, Ra and smooth S form) was investigated by biophysical means and was related to the peptides' biological activities. Both peptides exhibit high antibacterial activity against the three strains of Salmonella enterica differing in the LPS chemotype. Lauryl-LF11 has one order of magnitude higher activity against Re-type, but activity against Ra- and S-type bacteria is comparable with that of LF11. The alkyl derivative peptide lauryl-LF11 shows a much stronger inhibition of the LPS-induced cytokine induction in human mononuclear cells than LF11. Although peptide-LPS interaction is essentially of electrostatic nature, the lauryl-modified peptide displays a strong hydrophobic component. Such a feature might then explain the fact that saturation of the peptide binding takes place at a much lower peptide/LPS ratio for LF11 than for lauryl-LF11, and that an overcompensation of the negative LPS backbone charges is observed for lauryl-LF11. The influence of LF11 on the gel-to-liquid-crystalline phase-transition of LPS is negligible for LPS Re, but clearly fluidizing for LPS Ra. In contrast, lauryl-LF11 causes a cholesterol-like effect in the two chemotypes, fluidizing in the gel and rigidifying of the hydrocarbon chains in the liquid-crystalline phase. Both peptides convert the mixed unilamellar/non-lamellar aggregate structure of lipid A, the 'endotoxic principle' of LPS, into a multilamellar one. These data contribute to the understanding of the mechanisms of the peptide-mediated neutralization of endotoxin and effect of lipid modification of peptides.
Figures


References
-
- Vogel H. J., Schibli D. J., Jing W., Lohmeier-Vogel E. M., Epand R. F., Epand R. M. Towards a structure–function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell Biol. 2002;80:49–63. - PubMed
-
- Baynes R. D., Bezwoda W. R. Lactoferrin and the inflammatory response. In: Hutchens T. W., Lönnerdal B., Rumball S., editors. Lactoferrin: Structure and Function. New York: Plenum Press; 1994.
-
- Wakabayashi H., Takase M., Tomita M. Lactoferricin derived from milk protein lactoferrin. Curr. Pharm. Des. 2003;9:1277–1287. - PubMed
-
- Zähringer U., Lindner B., Rietschel E. T. Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharides. Adv. Carbohydr. Chem. Biochem. 1994;50:211–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

