The cyclooxygenases
- PMID: 15345041
- PMCID: PMC522864
- DOI: 10.1186/gb-2004-5-9-241
The cyclooxygenases
Abstract
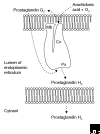
Cyclooxygenases (COXs) catalyze the rate-limiting step in the production of prostaglandins, bioactive compounds involved in processes such as fever and sensitivity to pain, and are the target of aspirin-like drugs. COX genes have been cloned from coral, tunicates and vertebrates, and in all the phyla where they are found, there are two genes encoding two COX isoenzymes; it is unclear whether these genes arose from an early single duplication event or from multiple independent duplications in evolution. The intron-exon arrangement of COX genes is completely conserved in vertebrates and mostly conserved in all species. Exon boundaries largely define the four functional domains of the encoded protein: the amino-terminal hydrophobic signal peptide, the dimerization domain, the membrane-binding domain, and the catalytic domain. The catalytic domain of each enzyme contains distinct peroxidase and cyclooxygenase active sites; COXs are classified as members of the myeloperoxidase family. All COXs are homodimers and monotopic membrane proteins (inserted into only one leaflet of the membrane), and they appear to be targeted to the lumenal membrane of the endoplasmic reticulum, where they are N-glycosylated. In mammals, the two COX genes encode a constitutive isoenzyme (COX-1) and an inducible isoenzyme (COX-2); both are of significant pharmacological importance.
Figures

References
-
- Daiyasu H, Toh H. Molecular evolution of the myeloperoxidase family. J Mol Evol. 2000;51:433–445. An evolutionary analysis of the myeloperoxidases. - PubMed
-
- Hemler M, Lands WEM, Smith WL. Purification of the cyclooxygenase that forms prostaglandins: demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976;251:5575–5579. The first isolation and purification of cyclooxygenase from sheep seminal vesicles. - PubMed
-
- Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976;251:2629–2636. The first isolation and purification of cyclooxygenase enzyme from bovine seminal vesicles. - PubMed
-
- Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun. 1989;165:888–894. The cloning of human cyclooxygenase-1 (COX-1) - PubMed
-
- Merlie JP, Fagan D, Mudd J, Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988;263:3550–3553. The cloning of the first cyclooxygenase gene from sheep. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

