Valproic acid teratogenicity: a toxicogenomics approach
- PMID: 15345369
- PMCID: PMC1277116
- DOI: 10.1289/txg.7034
Valproic acid teratogenicity: a toxicogenomics approach
Abstract
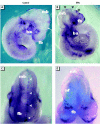
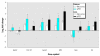
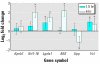
Embryonic development is a highly coordinated set of processes that depend on hierarchies of signaling and gene regulatory networks, and the disruption of such networks may underlie many cases of chemically induced birth defects. The antiepileptic drug valproic acid (VPA) is a potent inducer of neural tube defects (NTDs) in human and mouse embryos. As with many other developmental toxicants however, the mechanism of VPA teratogenicity is unknown. Using microarray analysis, we compared the global gene expression responses to VPA in mouse embryos during the critical stages of teratogen action in vivo with those in cultured P19 embryocarcinoma cells in vitro. Among the identified VPA-responsive genes, some have been associated previously with NTDs or VPA effects [vinculin, metallothioneins 1 and 2 (Mt1, Mt2), keratin 1-18 (Krt1-18)], whereas others provide novel putative VPA targets, some of which are associated with processes relevant to neural tube formation and closure [transgelin 2 (Tagln2), thyroid hormone receptor interacting protein 6, galectin-1 (Lgals1), inhibitor of DNA binding 1 (Idb1), fatty acid synthase (Fasn), annexins A5 and A11 (Anxa5, Anxa11)], or with VPA effects or known molecular actions of VPA (Lgals1, Mt1, Mt2, Id1, Fasn, Anxa5, Anxa11, Krt1-18). A subset of genes with a transcriptional response to VPA that is similar in embryos and the cell model can be evaluated as potential biomarkers for VPA-induced teratogenicity that could be exploited directly in P19 cell-based in vitro assays. As several of the identified genes may be activated or repressed through a pathway of histone deacetylase (HDAC) inhibition and specificity protein 1 activation, our data support a role of HDAC as an important molecular target of VPA action in vivo.
Figures


Similar articles
-
Short-time gene expression response to valproic acid and valproic acid analogs in mouse embryonic stem cells.Toxicol Sci. 2011 Jun;121(2):328-42. doi: 10.1093/toxsci/kfr070. Epub 2011 Apr 6. Toxicol Sci. 2011. PMID: 21427059
-
Valproic acid-induced deregulation in vitro of genes associated in vivo with neural tube defects.Toxicol Sci. 2009 Mar;108(1):132-48. doi: 10.1093/toxsci/kfp002. Epub 2009 Jan 8. Toxicol Sci. 2009. PMID: 19136453
-
New molecular bioassays for the estimation of the teratogenic potency of valproic acid derivatives in vitro: activation of the peroxisomal proliferator-activated receptor (PPARdelta).Toxicol Appl Pharmacol. 1999 Nov 1;160(3):238-49. doi: 10.1006/taap.1999.8770. Toxicol Appl Pharmacol. 1999. PMID: 10544058
-
Valproic acid-induced neural tube defects in mouse and human: aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms.Pharmacol Toxicol. 1991 Nov;69(5):310-21. doi: 10.1111/j.1600-0773.1991.tb01303.x. Pharmacol Toxicol. 1991. PMID: 1803343 Review.
-
Valproic acid in pregnancy: how much are we endangering the embryo and fetus?Reprod Toxicol. 2009 Jul;28(1):1-10. doi: 10.1016/j.reprotox.2009.02.014. Epub 2009 Mar 13. Reprod Toxicol. 2009. PMID: 19490988 Review.
Cited by
-
A human pluripotent stem cell platform for assessing developmental neural toxicity screening.Stem Cell Res Ther. 2013;4 Suppl 1(Suppl 1):S12. doi: 10.1186/scrt373. Epub 2013 Dec 20. Stem Cell Res Ther. 2013. PMID: 24565336 Free PMC article. Review.
-
Interaction networks of lithium and valproate molecular targets reveal a striking enrichment of apoptosis functional clusters and neurotrophin signaling.Pharmacogenomics J. 2012 Aug;12(4):328-41. doi: 10.1038/tpj.2011.9. Epub 2011 Mar 8. Pharmacogenomics J. 2012. PMID: 21383773 Free PMC article.
-
Valproic Acid in Pregnancy Revisited: Neurobehavioral, Biochemical and Molecular Changes Affecting the Embryo and Fetus in Humans and in Animals: A Narrative Review.Int J Mol Sci. 2023 Dec 27;25(1):390. doi: 10.3390/ijms25010390. Int J Mol Sci. 2023. PMID: 38203562 Free PMC article. Review.
-
Genetic and maternal effects on valproic acid teratogenesis in C57BL/6J and DBA/2J mice.Toxicol Sci. 2010 Aug;116(2):632-9. doi: 10.1093/toxsci/kfq140. Epub 2010 May 10. Toxicol Sci. 2010. PMID: 20457659 Free PMC article.
-
Valproic acid use is associated with diminished risk of contracting COVID-19, and diminished disease severity: Epidemiologic and in vitro analysis reveal mechanistic insights.PLoS One. 2024 Aug 2;19(8):e0307154. doi: 10.1371/journal.pone.0307154. eCollection 2024. PLoS One. 2024. PMID: 39093886 Free PMC article.
References
-
- Aardema MJ, MacGregor JT. Toxicology and genetic toxicology in the new era of “toxicogenomics”: impact of “-omics” technologies. Mutat Res. 2002;499(1):13–25. - PubMed
-
- Al Deeb S, Al Moutaery K, Arshaduddin M, Tariq M. Vitamin E decreases valproic acid induced neural tube defects in mice. Neurosci Lett. 2000;292(3):179–182. - PubMed
-
- Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59(1):95–104. - PubMed
-
- Arinze IJ, Kawai Y. Sp family of transcription factors is involved in valproic acid-induced expression of galphai2. J Biol Chem. 2003;278(20):17785–17791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

