Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance
- PMID: 15345415
- PMCID: PMC520879
- DOI: 10.1128/AEM.70.9.5315-5322.2004
Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance
Abstract
Amino acid decarboxylation-antiporter reactions are one of the most important systems for maintaining intracellular pH between physiological limits under acid stress. We analyzed the Lactobacillus acidophilus NCFM complete genome sequence and selected four open reading frames with similarities to genes involved with decarboxylation reactions involved in acid tolerance in several microorganisms. Putative genes encoding an ornithine decarboxylase, an amino acid permease, a glutamate gamma-aminobutyrate antiporter, and a transcriptional regulator were disrupted by insertional inactivation. The ability of L. acidophilus to survive low-pH conditions, such as those encountered in the stomach or fermented dairy foods, was investigated and compared to the abilities of early- and late-stationary-phase cells of the mutants by challenging them with a variety of acidic conditions. All of the integrants were more sensitive to low pH than the parental strain. Interestingly, each integrant also exhibited an adaptive acid response during logarithmic growth, indicating that multiple mechanisms are present and orchestrated in L. acidophilus in response to acid challenge.
Figures
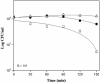




 , NCK1684 (ornithine decarboxylase); ⊞, NCK1682 (amino acid permease).
, NCK1684 (ornithine decarboxylase); ⊞, NCK1682 (amino acid permease).
References
-
- Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

