Role of hexose transport in control of glycolytic flux in Saccharomyces cerevisiae
- PMID: 15345416
- PMCID: PMC520882
- DOI: 10.1128/AEM.70.9.5323-5330.2004
Role of hexose transport in control of glycolytic flux in Saccharomyces cerevisiae
Abstract
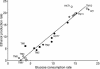
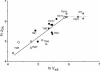
The yeast Saccharomyces cerevisiae predominantly ferments glucose to ethanol at high external glucose concentrations, irrespective of the presence of oxygen. In contrast, at low external glucose concentrations and in the presence of oxygen, as in a glucose-limited chemostat, no ethanol is produced. The importance of the external glucose concentration suggests a central role for the affinity and maximal transport rates of yeast's glucose transporters in the control of ethanol production. Here we present a series of strains producing functional chimeras between the hexose transporters Hxt1 and Hxt7, each of which has distinct glucose transport characteristics. The strains display a range of decreasing glycolytic rates resulting in a proportional decrease in ethanol production. Using these strains, we show for the first time that at high glucose levels, the glucose uptake capacity of wild-type S. cerevisiae does not control glycolytic flux during exponential batch growth. In contrast, our chimeric Hxt transporters control the rate of glycolysis to a high degree. Strains whose glucose uptake is mediated by these chimeric transporters will undoubtedly provide a powerful tool with which to examine in detail the mechanism underlying the switch between fermentation and respiration in S. cerevisiae and will provide new tools for the control of industrial fermentations.
Figures
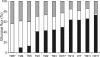

References
-
- Bisson, L. F., D. M. Coons, A. L. Kruckeberg, and D. A. Lewis. 1993. Yeast sugar transporters. Crit. Rev. Biochem. Mol. Biol. 28:259-308. - PubMed
-
- Blazquez, M. A., R. Lagunas, C. Gancedo, and J. M. Gancedo. 1993. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 329:51-54. - PubMed
-
- Boeke, J. D., F. LaCroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. - PubMed
-
- Boles, E., and C. P. Hollenberg. 1997. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 21:85-111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

