Microbial species involved in production of 1,2-sn-diacylglycerol and effects of phosphatidylcholine on human fecal microbiota
- PMID: 15345455
- PMCID: PMC520862
- DOI: 10.1128/AEM.70.9.5659-5666.2004
Microbial species involved in production of 1,2-sn-diacylglycerol and effects of phosphatidylcholine on human fecal microbiota
Abstract
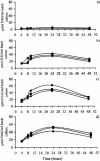
1,2-sn-Diacylglycerols (DAGs) are activators of protein kinase C (PKC), which is involved in the regulation of colonic mucosal proliferation. Extracellular DAG has been shown to stimulate the growth of cancer cell lines in vitro and may therefore play an important role in tumor promotion. DAG has been detected in human fecal extracts and is thought to be of microbial origin. Hitherto, no attempts have been made to identify the predominant fecal bacterial species involved in its production. We therefore used anaerobic batch culture systems to determine whether fecal bacteria could utilize phosphatidylcholine (0.5% [wt/vol]) to produce DAG. Production was found to be dependent upon the presence of the substrate and was enhanced in the presence of high concentrations of deoxycholate (5 and 10 mM) in the growth medium. Moreover, its production increased with the pH, and large inter- and intraindividual variations were observed between cultures seeded with inocula from different individuals. Clostridia and Escherichia coli multiplied in the fermentation systems, indicating their involvement in phosphatidylcholine metabolism. On the other hand, there was a significant decrease in the number of Bifidobacterium spp. in the presence of phosphatidylcholine. Pure-culture experiments showed that 10 of the 12 strains yielding the highest DAG levels (>50 nmol/ml) were isolated from batch culture enrichments run at pH 8.5. We found that the strains capable of producing large amounts of DAG were predominantly Clostridium bifermentans (8 of 12), followed by Escherichia coli (2 of 12). Interestingly, one DAG-producing strain was Bifidobacterium infantis, which is often considered a beneficial gut microorganism. Our results have provided further evidence that fecal bacteria can produce DAG and that specific bacterial groups are involved in this process. Future strategies to reduce DAG formation in the gut should target these species.
Figures


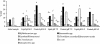
Similar articles
-
In vitro effects of phosphatidylcholine and transgalactooligosaccharides on the production of 1,2-sn-diacylglycerol by Bifidobacterium longum biovar infantis.J Appl Microbiol. 2008 Nov;105(5):1678-85. doi: 10.1111/j.1365-2672.2008.03886.x. Epub 2008 Oct 1. J Appl Microbiol. 2008. PMID: 18828790
-
Production of diacylglycerol, an activator of protein kinase C, by human intestinal microflora.Cancer Res. 1990 Jun 15;50(12):3595-9. Cancer Res. 1990. PMID: 2340507
-
Calcium reduces the increased fecal 1,2-sn-diacylglycerol content in intestinal bypass patients: a possible mechanism for altering colonic hyperproliferation.Cancer Res. 1994 Mar 1;54(5):1216-9. Cancer Res. 1994. PMID: 8118809 Clinical Trial.
-
Dietary fat, fiber, and carcinogen alter fecal diacylglycerol composition and mass.Cancer Res. 1995 Jun 1;55(11):2293-8. Cancer Res. 1995. PMID: 7757978
-
Selective production of functional sn-1,3-diacylglycerol by microbial lipases: A comprehensive review.Food Chem. 2025 Jul 30;481:144017. doi: 10.1016/j.foodchem.2025.144017. Epub 2025 Mar 27. Food Chem. 2025. PMID: 40179503 Review.
Cited by
-
Fecal microbiome and metabolome differ in healthy and food-allergic twins.J Clin Invest. 2021 Jan 19;131(2):e141935. doi: 10.1172/JCI141935. J Clin Invest. 2021. PMID: 33463536 Free PMC article. Clinical Trial.
-
Fermentative Production of Diacylglycerol by Endophytic Fungi Screened from Taxus chinensis var. mairei.Foods. 2023 Jan 14;12(2):399. doi: 10.3390/foods12020399. Foods. 2023. PMID: 36673491 Free PMC article.
-
Clostridium Scindens Protects Against Vancomycin-Induced Cholestasis and Liver Fibrosis by Activating Intestinal FXR-FGF15/19 Signaling.Adv Sci (Weinh). 2025 Feb;12(5):e2406445. doi: 10.1002/advs.202406445. Epub 2024 Dec 16. Adv Sci (Weinh). 2025. PMID: 39680750 Free PMC article.
-
Precision Dietary Intervention: Gut Microbiome and Meta-metabolome as Functional Readouts.Phenomics. 2025 Feb 14;5(1):23-50. doi: 10.1007/s43657-024-00193-7. eCollection 2025 Feb. Phenomics. 2025. PMID: 40313608 Review.
-
Integrated meta-omics reveals the regulatory landscape involved in lipid metabolism between pig breeds.Microbiome. 2024 Feb 20;12(1):33. doi: 10.1186/s40168-023-01743-3. Microbiome. 2024. PMID: 38374121 Free PMC article.
References
-
- Ali, S. S., and A. Kuksis. 1967. Excretion of phospholipids by men on high fat diets. Can. J. Biochem. 45:703-714. - PubMed
-
- Archer, R. H., I. S. Maddox, and R. Chong. 1982. Transformation of cholic acid by Clostridium bifermentans. J. Appl. Bacteriol. 52:49-56.
-
- Barsukov, L. I., V. I. Kulikov, I. M. Simakova, G. V. Tikhonova, D. N. Ostrovskii, and L. D. Bergelson. 1978. Manipulation of phospholipid composition of membranes with the aid of lipid exchange proteins. Eur. J. Biochem. 90:331-336. - PubMed
-
- Bingham, S. A. 1996. Epidemiology and mechanisms relating diet to risk of colorectal cancer. Nutr. Res. Rev. 9:197-239. - PubMed
-
- Bligh, E. G., and W. J. A. Dyer. 1964. A rapid method of total lipid extraction. Can. J. Biochem. 37:911-917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

